Introduction
Transition between the larger airways and interstitial space, the small airways normally offer < 20% of total resistance below the larynx and were once considered a silent zone [1]. This quietude, however, has been rattled, with more and more documentary evidence that there is definite and early involvement of small airways which actively contribute to enhanced airway hyper responsiveness in asthma and COPD [2]. In COPD, small airways involvement leads to an increase in airflow resistance from 4 to 40 folds [3,4].
COPD is an unrecognized epidemic; it is often misdiagnosed or under assessed. To begin with, patients with COPD as well as asthma tend to disregard their symptoms of cough with expectoration as inconsequential. In fact, exertional dyspnoea may not even be acknowledged in relatively sedentary individuals. Studies have even concluded that the exertional dyspnoea which is typically seen in mild COPD is due to possible distal airway obstruction [5]. Even when evaluated by general practitioners, these disorders may go undetected or under treated, sometimes dismissing it off as a simple bronchitis [6]. To complicate matters further, the major site of increased resistance in COPD is the small airways, which are more difficult to assess and treat [7]. Another challenge that is faced in some patients in the early stages is that it may be impossible to differentiate them from bronchiolitis and a diagnosis may evolve only by the time the disease has progressed. This article is aimed at serving a dual purpose. First, to highlight the need for early recognition of distal airways involvement and the airway remodeling that takes place in both asthma and COPD [8]. The second purpose is to review the role of imaging to help pick up this involvement early.
Pulmonary Function Techniques to Assess Small Airway Obstruction
Conventional spirometry: It is a tool used to diagnose airway obstruction and Maximum Mid-Expiratory Flow (MMEF) or FEF25-75 is considered to be an indicator of small airways. However, it is highly dependent of Forced Vital Capacity (FVC) and may give paradoxical results after bronchodilation. Therefore, it needs to be obtained at the same FVC.
Plethysmography: It measures residual volume and airway resistance as well as RV/TLC. It is simple to perform, sensitive and easily available however, it has low specificity for small airway changes and procedure is time consuming [9].
Inert gas washout: This is relatively novel technique and is sensitive in demonstrating early airway changes as well as can distinguish between proximal and distal airway obstruction however, it is still limited for research purposes.
Impulse oscillometry: Another method that can diagnose small airway obstruction is Impulse oscillometry. Equipment both for Impulse Oscillometry and Isovolumetric MMEF are not commonly available therefore, imaging becomes an important modality to identify small airway obstruction.
High Resolution CT Chest for Assessing Small Airways
Protocol: High resolution CT (HRCT) is an imaging technique which includes acquiring thin slices of the lungs and reconstruction is done using sharp algorithm for example bone algorithm. HRCT is widely requested for assessment of interstitial lung diseases owing to better assessment of interstitial pattern and early identification of parenchymal and interstitial changes in the lungs which ultimately improves patient care.
There are two basic techniques of acquiring HRCT chest. First one is axial or non-volumetric technique or step and shoot technique which includes acquiring images at a gap of 1 or 2 cm and is particularly useful in diffuse lung diseases. The advantage is lesser radiation dose to the patient however, coronal and sagittal reconstructions are difficult to evaluate due to step artefacts.
Second is Volumetric HRCT which includes obtaining continuous images of the lungs and thus, provides better opportunity to completely evaluate the lungs as well as better sagittal and coronal reformats. However, the radiation dose to the patient is higher as compared to the axial HRCT technique [10].
Basic HRCT chest protocol includes a slice thickness of approximately 0.625-1.25 mm, kV= 120, mAs =100-200, collimation= 1.5-3 mm, scan time= 0.1 sec, high spatial resolution reconstruction algorithm and a matrix size of 768 x 768 or largest possible with a field of view approximately 35 cm. Scanning is usually performed in supine position except in cases of suspected early interstitial changes and to differentiate between early interstitial changes and dependent density. Full inspiration during the time of scan is routinely recommended, expiratory scans are performed in suspected airway obstructive diseases to assess air trapping.
HRCT chest is widely used for the assessment of airway diseases. It is helpful in demonstrating air trapping and airways related changes. It is simple, easy to perform, rapid technique however radiation and difficulty in direct visualization of small airways is a limitation [10]. The direct and indirect signs of small airway obstruction in HRCT are discussed below (please refer discussion section).
Future Trends in Imaging of Small Airways
Recent trends are towards the emerging role of MRI (Hyperpolarized MRI) and nuclear medicine including Single Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET) however, their roles are largely limited for research purposes and more comprehensive role of these imaging techniques shall be evident in the upcoming years.
Clinical Implications of Small Airway Disease
In order to understand the pathological aspects of small airways disease, it is imperative to first revise the anatomy of the same. The human respiratory tract is divided into 23 generations by dichotomously branching airways beginning at the trachea and culminating in the acinar sacs. As per the Hess and Murray law, the diameter of the daughter airways reduces by a factor of 0.79 compared to the parent airway till atleast the respiratory bronchiole [11,12]. This characteristic arrangement of the airways in the form of multiple parallel air conducting cylinders is responsible for a host of consequences. Apart from the obvious function of conducting air to the peripheral parts of the lungs, the dichotomy aids gas exchange as well; the great mass of branching bronchioles increase the cross-sectional area exponentially; from a meager 2.5 cm2 at the level of the trachea to nearly 300 cm2 at the level of the acinar airways. This produces a drop in the airflow velocity by nearly 100 times from the trachea to the acini. Moreover, with regards to the smaller bronchioles, airway diameter reduces by a factor more than 0.79 which add to the increased distensibility of the bronchioles during respiratory excursions helps in reducing the resistance to airflow [13]. However, this segment of the airways is also prone to obstructive processes due to the small size. The obstruction of small airways occurs through varied mechanisms, including mucus impaction, reduction in airway diameter from inflammatory infiltrates, smooth muscle hypertrophy, or airway wall thickening. Also, loss of structural airway supports may enhance collapsibility of airways. More than one of these pathological processes are frequently in play in case of COPD and asthma [14].
Assessment of small airways involvement is often challenging and is presently effected through modalities measuring physiological parameters such as air flows, resistance, distribution of ventilation and air trapping and radiological assessment [15]. The conventional pulmonary function tests such as Forced Expiratory Volume in one second (FEV1), FEF25-75, FEV1/FVC, FEV3/FVC and FEV/SVC are reproducible and widely accepted but are non specific and insensitive for early disease. Plethysmographic measurement of residual volumes and airways resistance are sensitive but non specific for early airways disease and labour intensive. Impulse oscillometry and gas washout methods are relatively more specific but not widely available and require specialized equipment. HRCT is presently the most widely used radiological modality in assessing the lung in small airways disease. Although the said segment of airways is not directly visualized, various estimates can be derived. Also, available for assessment are magnetic resonance and radionuclide scanning techniques [15,16].
Inhaled Corticosteroids (ICS) are frequently used in the treatment of COPD. Hogg et al., investigated small airways in COPD in relation to its severity and concluded that inflammation is an important feature of small airway involvement [16]. Moreover, inflammation involves not just the small airways but also the lung parenchyma. This fact is probably responsible for the limited role of ICS in COPD, since large-particle ICS are deposited mainly in the central airways, while the small airways and parenchyma stay largely unexposed to the drug. The availability of new devices such as those driven by hydrofluoroalkanes (HFAs) allow drugs to better target the small airways and have been shown to improve disease severity in terms of non invasive markers of disease activity and ventilator parameters [16,17]. This may have important clinical implications [14–16]. Various studies in asthma have shown a larger improvement in small airway function and inflammation with small particle aerosols than with larger particle aerosols [18]. This may be clinically relevant, especially for patients with uncontrolled asthma not responding to large particle aerosols. This opens up a possibility that an intervention done at an early stage, where small airways are chiefly involved could alter the course of the disease. This also has the potential to provide symptomatic relief and improve quality of life in those patients in whom no respite is achieved despite maximal available pharmacotherapy. Hence, an early recognition of small airway involvement during this potential therapeutic window may prevent complications and delay decline of lung function in both COPD and asthma.
Discussion
The key to appreciate the pathology of small airway disease lies in thorough understanding of the functional organization of the human airways. The airways consist of approximately 23 generations of dichotomously branching tubes from the trachea to the alveoli. The first 15 generations of airways are called the conducting airways and take no part in gas exchange. Beyond this region lie the respiratory bronchioles which have occasional alveoli budding from them. These continue to divide until they reach the alveolar sacs. These airways take part in gas exchange and comprise the acinar airways. The small airways refer to those airways that are less than 2 mm in diameter. [19].
It is difficult to assess isolated small airway obstruction that may be seen in early COPD. Although Forced expiratory flow during mid expiration (FEF25-75%) does reflect resistance encountered in small airways but this parameter is not uniformly reproducible and is highly dependent on FVC. Reversibility in this parameter also requires to be interpreted secondarily to that seen in FEV1. Also, it is estimated that obstruction of 75% of small airways is required before significant airflow limitation can be detected by routine PFT. Thus these values, though readily available, have their own limitation and need to be interpreted with caution [14]. However, as discussed above, with the potential that recognition of small airway obstruction may have on disease outcome, it becomes pertinent to look at other modalities that can identify this early.
Imaging provides a novel noninvasive method to assess small airways in obstructive pulmonary disease. HRCT not only provides a very simple and direct assessment of large and medium airways (diameter >2-2.5 mm) but also gives an assessment of smaller airways. Normally distal airways are not visible on CT because the thickness of their walls (0.6-1 mm) is below the CT resolution threshold of 1 mm. Nevertheless, in case of distal airway obstruction, CT provides us with useful direct signs which are an outcome of direct visualization of the diseased small airways or certain indirect signs which are due to abnormalities in the parenchyma distal to the obstructed small airways [Table/Fig-1(a,b),2].
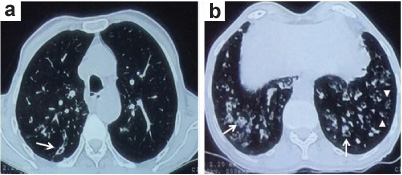
Contrast enhanced computed tomogram of the thorax showing; a) bronchiolar wall thickening and bronchiolectasis (arrows) representing the small airway involvement and; b) multiple subtle linear branching opacities in the periphery (arrowheads) seen as a direct sign of small airway disease.
Direct and indirect radiological signs of small airway disease seen on CT thorax.
| Direct Signs | Indirect Signs |
|---|
| Centrilobular nodules | Mosaic attenuation |
| Tree-in-bud opacities | Air trapping |
| Sub segmental atelectasis |
| Wedge shaped ground glass areas |
| Cylindrical bronchiolectasis |
| Centrilobular emphysema |
| Mucoid impaction |
| Bronchiolar wall thickening |
CT Signs of Small Airway Obstruction
Direct Signs: Direct signs are visualization of diseased, filled or dilated bronchioles which are normally not visible on HRCT. Depending on the plane of scanning, they are seen as ill defined centrilobular nodules or well defined branching centrilobular nodules, popularly known as the “tree in bud” appearance [Table/Fig-3]. A lot of normal airways and blood vessels predominate in the central portion of lung architecture where such findings can at times be difficult to discern. However, in the lung periphery (within 1 cm from the pleural surface), these abnormal small airways have a distinctive appearance. Any ring like, tubular, nodular, linear or branching linear structure seen in this location is always abnormal. These may be seen as tiny branching subpleural opacities of high attenuation along the length or as nodular opacities in an axial plane which on tracing the contiguous 1 mm spiral scan, can be traced to a small airway [20,21]. However, “tree in bud” is not specific for small airway disease and can be seen in any condition that may lead to mucoid impaction in small airways like acute bronchiolitis, tuberculosis, infiltrative lung diseases etc., or in isolation [21,22].
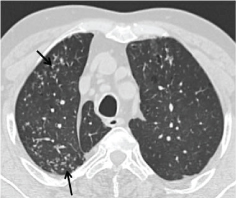
High resolution CT thorax showing sub pleural branching structures represent mucus filled small airways tree- in- bud appearance (arrows).
Indirect Signs: Indirect signs of small airway disease are due to secondary changes seen in the lung parenchyma distal to a diseased small airway. A mosaic pattern of attenuation on inspiratory scan and air trapping at expiratory scan [Table/Fig-4,5] are the most common findings. Lung parenchyma distal to the stenotic small airway remains hyperaerated giving rise to a patchy mosaic pattern when seen against areas where normal pulmonary architecture is preserved. When the disease process becomes diffuse and affects most or all of lung, this pattern can be difficult to recognize as abnormal. Hence, there is always a relative paucity of CT findings in comparison to the extent or severity of disease in small airways [21,23]. However, it must be noted that mosaic pattern and air trapping are not specific for asthma or COPD and were first described in bronchiolitis obliterans. Air trapping is universally present in all diseases affecting the distal airways and can normally be seen in healthy subjects and obese individuals with its prevalence increasing with age and smoking [24]. [Table/Fig-2] summarizes the direct and indirect signs of small airway disease on the HRCT.
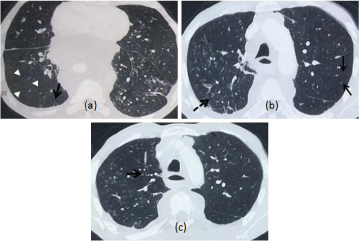
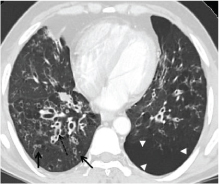
a) HRCT thorax of a patient with COPD displaying focal areas of hyperlucency suggestive of air trapping in the right lung (arrowheads) with linear and branching small peripherally located opacities (arrow) representing distal airways obstruction; b) Non contrast CT in a patient with severe COPD displaying diffuse involvement of small airways due to which the mosaic pattern is not appreciated and there is diffuse hypoattenuation of the lung (arrows). Also noted are the direct signs of nodular and linear branching opacities on the right side (dashed arrow); c) Bronchial wall thickening noted in the same patient.
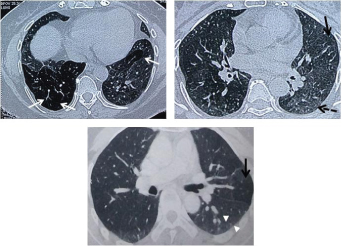
HRCT thorax images: a) Expiratory scan of an asthmatic patient showing segmental areas of air-trapping (arrow) seen as hyperlucent areas bilaterally; b) Same patient having lobular areas of air trapping on the left side also (arrow). A few scattered centrilobular nodules peripherally (dashed arrow) representing small airway obstruction; c) Expiratory scans of a poorly-controlled asthmatic patient showing patchy lobular areas of air trapping (arrow). Note the thin linear branching opacities seen in the periphery representing obliteration of small airways (arrowheads).
Several features differentiate normal air trapping from that related to bronchiolar disease. Normal air trapping is confined to a few lobules and most marked in bases and dependent areas in lungs, whereas in distal airway disease, it affects multiple lobules, segments or lobes and extends beyond the lung bases and dependent areas and is always more prominent in expiratory scans. Air trapping also has to be differentiated from patchy ground glass attenuation. An expiratory scan helps differentiate the two. In an expiratory scan, air trapping becomes more prominent since the lighter areas of lung increases in attenuation on expiration and the darker abnormal lung (air trapped area) does not. Patchy ground glass areas on the other hand do not become more prominent on expiration, rather they stand out less. The presence of vascular attenuation in the darker areas also gives a helpful clue to determine air trapping [20,23].
The other indirect signs include a sub segmental atelectasis which occurs when the lung parenchyma distal to an obstructed small airway collapses. Wedge shaped areas of ground glass are seen which are very typical of small airway disease in asthma. Cylindrical bronchiolectasis, bronchiolar wall thickening, mucoid impaction and centrilobular emphysema [Table/Fig-6] may be other accompanying findings [20].
HRCT thorax showing bilateral bronchiectasis (dashed arrow) with bronchiolectasis with peripheral branching and linear densities (arrows) and associated emphysema (arrowheads) in the left lung suggestive of small airway involvement in a patient with COPD.
While, the role of quantitative HRCT in COPD is not yet clear, the same may not only be able to determine air trapping but also identify the high risk group with severe disease in asthmatic patients, as seen in a recent study [25,26]. In this, air trapping was defined as the percentage of lung less than 850 HU on expiratory CT. Randomized studies evaluating HRCT findings after metacholine induced bronchoconstriction and salbutamol induced bronchodilation & comparative studies between conventional Beclomethasone Dipropionate (BDP) and Hydrofluoro Alkane Beclomethasone Dipropionate (HFA-BDP)-extra fine particles, have been carried out [27]. Extra fine HFA-BDP particles are known to be deposited in distal airways better as compared to the conventional BDP. These studies show a decrease in air trapping in patients treated with HFA-BDP and less increase in air trapping after methacholine challenge in patients proved with distal airway obstruction [28]. Semi quantitative HRCT can prove to be an adjunct and sometimes more sensitive to changes in distal airway than overt clinical response or conventional physiological studies [29,30]. However, despite the results obtained so far suggesting that this technique is promising, it is still costly, technically demanding and hampered by exposure of patients to radiation.
Impulse oscillometry is an effective and easy to perform, non invasive method of assessment of distal airway. Single breath hold or multiple breath nitrogen washout studies can also distinguish between ventilation inhomogeneity arising from distal versus proximal airways [14].
The other imaging tools which are still under the realm of research include Helium-MRI which allows assessment of destruction of ventilation and morphometry of distal airways. Diffusion images visualize movement of helium in peripheral air spaces, calculated by Apparent Diffusion Co-Efficient (ADC). SPECT and PET also have been tried but all these modalities are still largely restricted to research application and their role in clinical management is not established yet [30].
Conclusion
The small airways of the lung are commonly afflicted yet often overlooked section of the respiratory system. Although these segments may be involved in a host of pathological processes, their involvement is probably one of the primary pathologies in obstructive airway disorders like COPD. The clinical significance of recognizing this affliction stems from the fact that presently, recognition of small airway disease is poor among clinicians and to make matters worse, no direct assessment is possible. While a number of pulmonary function parameters can indicate towards the pathology, they have their own pros and cons and may not be practically applicable universally. The direct and indirect signs seen on the CT scan are a reliable alternative to these more cumbersome tests and could help the clinician diagnose and treat the disease early since the same is associated with significant socio economic costs. Moreover, the dictum of ‘one size fits all’ may not actually be true since it is now beginning to get clear that the presently available inhalational therapy for COPD targets only the large airways while the disease continues to smolder in the smaller airways and lung parenchyma leading to an inexorable decline in lung function. Therefore, better recognition of small airway disease as a distinct pathological process holds the promise of a more site specific and targeted therapy.
[1]. Macklem PT, Mead J, Resistance of central and peripheral airways measured by a retrograde catheterJ Appl Physiol 1967 22:395-401. [Google Scholar]
[2]. Lipworth B, Manoharan A, Anderson W, Unlocking the quiet zone: The small airway asthma phenotypeLancet Respir Med 2014 2:497-506. [Google Scholar]
[3]. Burgel PR, The role of small airways in obstructive airway diseasesEur Respir Rev 2011 20:23-33. [Google Scholar]
[4]. Kaminsky DA. Small airway physiology: Why should we care? [Monograph on the internet]. Pulmonary and Critical Care University of Vermont Burlington, Vermont, USA. [cited 2015 Apr 08]. Available from: http://www.thoracic.org.au/imagesDB/wysiwyg/D4-1B-0830-3DavidKaminsky.pdf [Google Scholar]
[5]. Contoli M, Bousquet J, Fabbri LM, Magnussen H, Rabe KF, Siafakas NM, The sreappraisalAllergy 2010 65:141-51. [Google Scholar]
[6]. Jindal SK, COPD: The unrecognised epidemic in IndiaJ Assoc Physicians India 2012 60(Suppl):S14-16. [Google Scholar]
[7]. Vijayan VK, Chronic obstructive pulmonary diseaseIndian J Med Res 2013 137:251-69. [Google Scholar]
[8]. Sköld CM, Remodeling in asthma and COPD–differences and similaritiesClin Respir J 2010 4(Suppl 1):S20-27. [Google Scholar]
[9]. McNulty W, Usmani OS, Technique of assessing small airway dysfunctionEuropean Clinical Respiratory Journal 2014 Vol 1(2014)incl Supplements [Google Scholar]
[10]. Sundaram b, Chugtai AR, Kazerooni EA, Multidetector high resolution computed tomography of the lungs: Protocols and applicationsJ Thorac Imaging 2010 :125-41. [Google Scholar]
[11]. Hess WR, Das Prinzip des kleinsten Kraftverbrauches im Dienste hämodynamischer ForschungArch Anat Physiol 1914 2:1-62. [Google Scholar]
[12]. Murray CD, The physiological principle of minimum work. I. The vascular system and the cost of bloodProc Nat Acad Sci USA 1926 12:207-14. [Google Scholar]
[13]. Ochs M, Weibel ER, Functional design of the human lung for gas exchange. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI (eds)Fishman’s Pulmonary Diseases and Disorders 2008 4th edUSATata McGraw Hill:23-70. [Google Scholar]
[14]. McNulty W, Usmani OS, Techniques of assessing small airways dysfunctionEur Clin Respir J 2014 1:25898 [Google Scholar]
[15]. van den Berge M, ten Hacken NH, Cohen J, Douma WR, Postma DS, Small airway disease in asthma and COPD: Clinical implicationsChest 2011 139:412-23. [Google Scholar]
[16]. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, The nature of small airway obstruction in chronic obstructive pulmonary diseaseN Engl J Med 2004 350:2645-53. [Google Scholar]
[17]. van Beurden WJ, Harff GA, Dekhuijzen PN, van der Poel-Smet SM, Smeenk FW, Effects of inhaled corticosteroids with different lung deposition on exhaled hydrogen peroxide in stable COPD patientsRespiration 2003 70:242-48. [Google Scholar]
[18]. John M, Bosse S, Oltmanns U, Schumacher A, Witt C, Effects of inhaled HFA beclomethasone on pulmonary function and symptoms in patients with chronic obstructive pulmonary diseaseRespir Med 2005 99:1418-24. [Google Scholar]
[19]. Usmani OS, Small airways dysfunction in asthma: Evaluation and management to improve asthma controlAllergy Asthma Immunol Res 2014 6:376-88. [Google Scholar]
[20]. Ranga V, Kleinerman J, Structure and function of small airways in health and diseaseArch Pathol Lab Med 1978 102:609-17. [Google Scholar]
[21]. Teel GS, Engeler CE, Tashijian JH, duCret RP, Imaging of small airway diseaseRadiographics 1996 16:27-41. [Google Scholar]
[22]. Miller WT Jr, Panosian JS, Causes and imaging patterns of tree-in-bud opacitiesChest 2013 144:1883-92. [Google Scholar]
[23]. Burgel PR, Bergeron A, de Blic J, Bonniaud P, Bourdin A, Chanez P, Small airways diseases, excluding asthma and COPD: An overviewEur Respir Rev 2013 22:131-47. [Google Scholar]
[24]. Sudhakar NJP, Eric JS, Imaging of Small Airway Disease (SAD)Radiol Clin North Am 2009 47:307-16. [Google Scholar]
[25]. Lee KW, Chung SY, Yang I, Lee Y, Ko EY, Park MJ, Correlation of ageing and smoking with air trapping at thin section CT of the lung in asymptomatic subjectsRadiology 2000 214:831-36. [Google Scholar]
[26]. Ostridge K, Wilkinson TM, Present and future utility of computed tomography scanning in the assessment and management of COPDEur Respir J 2016 48:216-28. [Google Scholar]
[27]. Busacker A, Newell D Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, A multivariate analysis of risk factors for the air trapping asthmatic phenotype as measured by quantitative CT analysisChest 2009 135:48-56. [Google Scholar]
[28]. Goldin JG, Tashkin DP, Kleerup EC, Greaser LE, Haywood UM, Sayre JW, Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasonedipropionate inhalation on small airways: Assessment with functional helical thin section computed tomographyJ Allergy Clin Immunol 1999 104:S258-67. [Google Scholar]
[29]. Tunon-de-Lara JM, Laurent F, Giraud V, Perez T, Aguilaniu B, Meziane H, Air trapping in mild and moderate asthma: Effect of inhaled corticosteroidsJ Allergy Clin Immunol 2007 119:583-90. [Google Scholar]
[30]. Laurent F, Tunon de Lara M, Assesment of imaging techniques for evaluating small airway disease in asthmaRev Mal Respir 2011 28:e7-10. [Google Scholar]