Introduction
Vulvovaginal Candidiasis (VVC) is a common medical health problem of adult women. It is most commonly caused by Candida albicans. But there is a change in fungal profile. Sabouraud’s Dextrose Agar (SDA) is the most common culture medium used where mixed fungal infection may be missed. It can be detected easily by using chromogenic culture medium.
Aim
To know the fungal profile of vulvovaginal candidiasis using Candida CHROMagar and antifungal susceptibility pattern in patients attending tertiary care hospital.
Materials and Methods
Culture confirmed cases of VVC presented at Department of Obstetrics and Gynaecology of Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India, from July 2015 to December 2015 were included in the cross-sectional study. Two high vaginal swabs were collected and inoculated on SDA and Candida CHROMagar (Hi-Media, Mumbai, India). After overnight incubation the colonies were counted and colour of the colonies were recorded from Candida CHROMagar. Candida spp. were identified by sugar fermentation and assimilation tests and other conventional tests. Antifungal susceptibility tests were performed by the disc diffusion method using fluconazole (25 μg) and voriconazole (1μg) as per the Clinical and Laboratory Standards Institute (CLSI - M44-A2) guidelines.
Results
A total of 50 culture confirmed (23.7%) cases were detected from 211 clinically suspected VVC cases. Candida glabrata (45.1%) was the most common isolate, followed by Candidatropicalis (23.5%), Candida albicans (17.6%), Candida krusei (9.8%) and Candida parapsilosis (3.9%). One mixed infection of C. glabrata and C. albicans was identified on Candida CHROMagar. Mixed fungal infection was observed in 2% of positive culture and 0.5% of VVC cases. The antifungal susceptibility testing revealed that 15.7% and 9.8% isolates of Candida spp. were resistant and Susceptible Dose Dependent (S-DD) respectively to fluconazole. The increase resistant against fluconazole was because of increased isolation of C. glabrata strains. All strains of Candida spp. were susceptible to voriconazole.
Conclusion
C. glabrata was the most common causative agent of VVC in a tertiary care hospital. Chromogenic culture medium facilitates detection of mixed fungal infection. In vitro susceptibility testing should be used to guide the treatment especially in cases of non-albicans Candidiasis.
Antifungal susceptibility testing, Candida CHROMagar, Fungal infection
Introduction
VVC is a gynaecological condition diagnosed in a large proportion of women presenting with a complaint of abnormal vaginal discharge. It is caused by overgrowth of Candida species in the vagina [1]. It is clinically characterized by curd like discharge, itching dyspareunia, dysuria, oedema and vulvovaginal erythema [1]. The manifestations of VVC may range from asymptomatic colonization to severe acute symptomatic infection. Although it has a wide clinical presentation but there is no sign or symptoms which is pathognomonic of VVC [2]. Therefore, laboratory support may be required for accurate diagnosis. Asymptomatic colonization with Candida species are found in 10%-20% of women [3]. The risk of VVC is high in women with diabetes mellitus, Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS), oral contraceptives, using broad spectrum antibiotics therapy, pregnant women and women involved in receptive oral sex [4–6]. During lifetime, about 75% of woman may experience at least one episode of VVC and second episode may experience by 40%-45% of initially infected women. Recurrent Vulvovaginal Candidiasis (RVVC) is defined as four or more episodes of symptomatic VVC within a year [7]. It has been observed that less than 5% of women with VVC may develop RVVC [7]. VVC is classified into two different categories - uncomplicated case and complicated case. Sporadic episodes of mild to moderate infections mostly caused by C. albicans among immunocompetent women are known as uncomplicated case. Complicated cases are cases of VVC caused by Non-albicansCandida (NAC)/severe infection/recurrent infection/VVC during pregnancy or VVC associated with immunosuppression or diabetes [8].
Most of the time there is only one causative agent of VVC. SDA is the most common culture medium. Different species of yeast may not be differentiated on SDA. Now commercial chromogenic medium are available which easily detect mixed fungal infection [9]. Candida albicans is considered as the most common causative agent. It is isolated in 85%-90% cases of VVC [10]. C. glabrata is usually the second most common pathogen detected in VVC followed by other NAC- Candida tropicalis, Candida parapsilosis and Candida krusei [11,12]. C. tropicalis was found to be a predominant isolate next to the Candida albicans in some of the Indian study [13]. Interestingly isolation of C. glabrata from India varied widely from the most common isolate (61.3%) to less than 2% [12–14]. VVC by NAC are gradually increasing as a causative agent [13]. The fungal profile of VVC varies from one geographical area to another. There should be a periodic study to know the fungal profile and antifungal susceptibility pattern of VVC. Very limited studies are available from India about the fungal profile of VVC using chromogenic culture medium. This study was undertaken to know the fungal profile of VVC using Candida CHROMagar and antifungal susceptibility pattern in our geographical location.
Materials and Methods
A total of 211 clinically suspected cases of VVC presented at Department of Obstetrics and Gynaecology, from July 2015 to December 2015 in a tertiary care hospital. Patients were from 16-68 years of age with mean age of 33.4 (±8.7) years. Culture confirmed cases of VVC were included in the study which was approved by the Institute Ethics Committee. There were no exclusion criteria. Two High Vaginal Swabs (HVS) were collected from each patient and sent to the Department of Microbiology. Gram’s stain was performed on one HVS and observed microscopically for the presence of pus cells and budding yeast cell etc. Another HVS from each patient was emulsified in 1 ml of sterile saline and 1 μl of the suspension was inoculated on to the SDA and Candida CHROMagar (Hi-Media, Mumbai, India). SDA and Candida CHROMagar plates were incubated at 25°C. After overnight incubation the colonies were counted and colour of the colonies were recorded from Candida CHROMagar. Growth was confirmed as yeast by doing Gram’s stain. Candida spp. were identified by conventional tests like germ tube test, morphology on corn meal agar, sugar fermentation and assimilation tests [15,16]. Antifungal susceptibility tests were performed by the disc diffusion method using fluconazole (25 μg) and voriconazole (1 μg) (Hi-Media, Mumbai, India), on Muller Hinton Agar (MHA) (Hi-Media, Mumbai, India) supplemented with 2% Glucose and Methylene Blue dye 0.5 μg/ml (GMB) as per the CLSI-M44-A2 guidelines [17]. The categorical variables were expressed as frequency and percentages and the continuous variables were expressed as mean with standard deviation.
Results
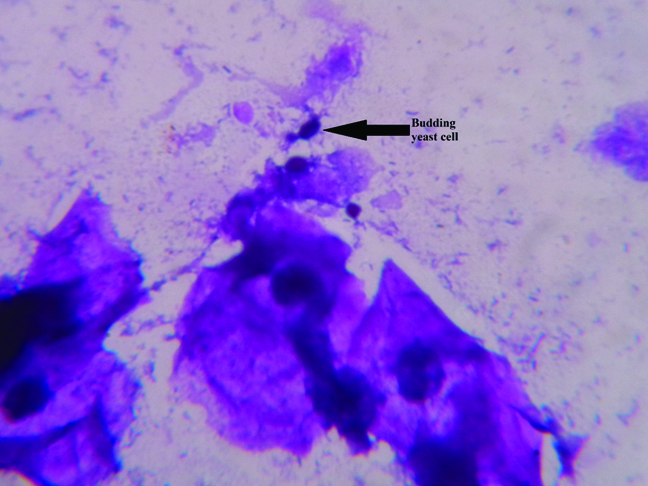
A total of 211 clinically suspected cases of VVC were presented during the study period. Fifty HVS (23.7%) yielded positive yeast on culture. All positive yeasts were inoculated on both the culture medium – SDA and Candida CHROMagar. Culture confirmed cases of VVC were from 21-50 years of age with mean age of 33.1 (±7.9) years. Twenty two patients (44%) were 21-30 years of age followed by 19 (38%) were 31-40 years and 9 were (18%) 41-50 years. It was observed that 16 (32%) patients were suffering from pelvic inflammatory disease, 1 (2%) had history of four years of infertility and only one patient (2%) was diabetic. Direct microscopic examinations of the Gram’s smear of HVS revealed budding yeast cell in only 7 (14%) cases [Table/Fig-1]. All of them yielded Candida on culture. Majority of high vaginal swabs – 40 (80%) revealed few pus cells (< 5 pus cells/HPF). Greater than 25 pus cells per HPF was observed in 2 (4%) HVSs, 10 to 15 pus cells/HPF were observed in 3 (6%) of them and 5 to 10 pus cells/HPF were seen in 5 (10%) HVSs.
Microscopic examination of high vaginal swab showing budding yeast cell (1000X).
SDA and Candida CHROMagar were able to isolate yeast in all positive cases [Table/Fig-2]. But one HVS yielded two Candida spp. which was appreciated on Candida CHROMagar. A total of 20 HVSs (40%) gave a colony count ≥ 105 colonies/ml, 20 swabs (40%) gave a count between 104-105 colonies/ml and 10 swabs (20%) showed colony count between 103-104 colonies/ml. A colony count of ≥ 105 colonies/ml were seen in both yeast isolates, in the mixed culture.
High vaginal swab streaked on; a) Sabouraud’s dextrose agar and; b) Candida CHROMagar where upper half showed yeast growth and lower half – no growth; c) Morphology and colour of yeast on Candida CHROM agar.
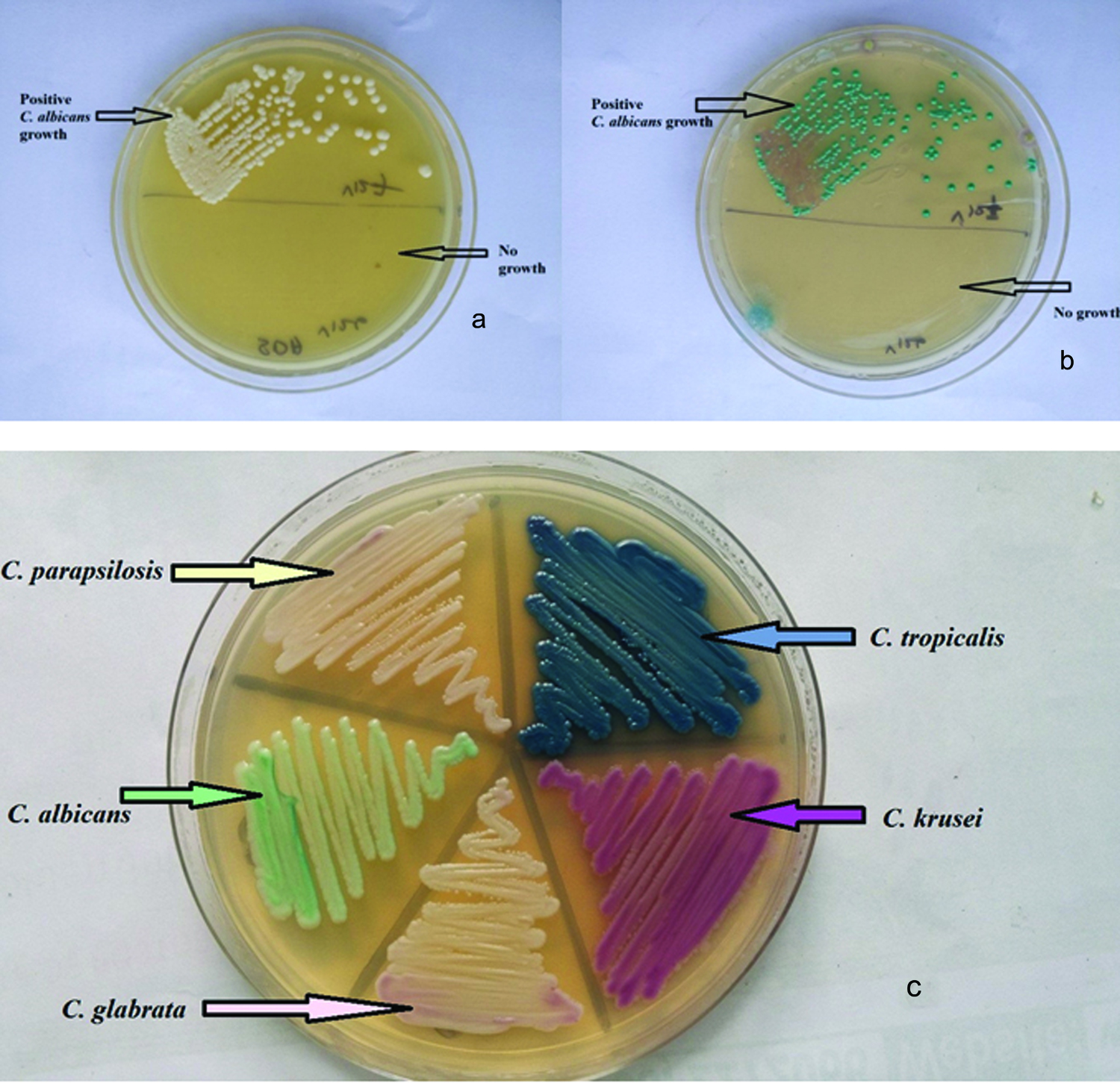
Candida glabrata was the most common isolate and observed in 23 cases (45.1%), followed by 12 cases (23.5%) of Candidatropicalis, 9 cases (17.6%) of Candida albicans, 5 cases (9.8%) of Candida krusei and 2 cases (3.9%) of Candida parapsilosis infections. One mixed infection of C. glabrata and C. albicans was identified. It was observed in 2% of positive yeast culture and 0.5% of VVC cases. All nine isolates of C. albicans were germ tube test positive.
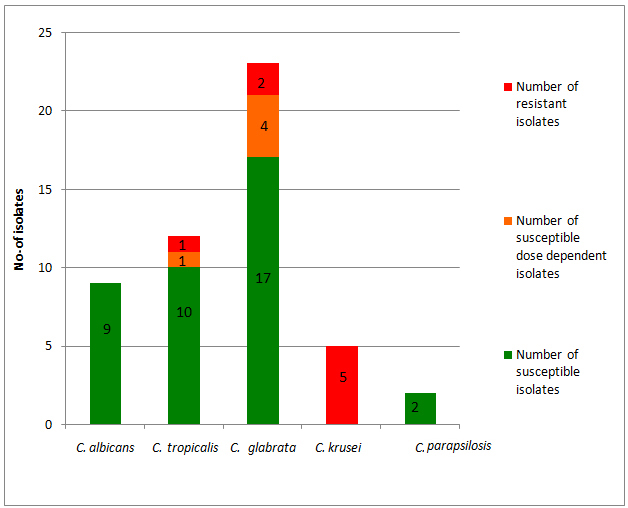
The antifungal susceptibility testing revealed that, 38 isolates (74.5%) were susceptible to fluconazole. A total of 8 (15.7%) isolates of Candida spp. were fluconazole resistant – which include all five strains of C. krusei, two strains of C. glabrata and single strain of C. tropicalis [Table/Fig-3]. All isolates of C. krusei were considered resistant to fluconazole as it is intrinsically resistant to fluconazole. There were five isolates (9.8%) of S-DD to fluconazole which include four of C. glabrata and one of C. tropicalis. All isolates of C. albicans and C. parapsilosis were susceptible to fluconazole. All Candida spp. were susceptible to voriconazole. In case of C. glabrata, 2 out of 23 isolates were resistant (8.7%) to fluconazole, 4 showed S-DD (17.4%), and other 17 were susceptible to fluconazole (73.9%). Among the C. tropicalis isolates 10 were susceptible (83.3%) and one isolate (8.3%) each of S-DD and resistant to fluconazole.
Antifungal Susceptibility Testing (AFST) of vaginal Candida isolates against fluconazole.
Discussion
VVC is a common public health problem of women presenting with a complaint of abnormal vaginal discharge. A total of 50 participants (23.7%) yielded positive yeast isolates from 211 clinically suspected cases of VVC which is in correlation with other studies [18–20]. It was predominantly seen in sexually active adult females and 44% of the participants were from 21 to 30 years of age in the study which is in correlation with other similar study [18,20]. Associated co-morbid condition and the risk factors were recoded from the requisition proforma on the first visit of the patient. It revealed only one diabetic patient. Risk factors would have been improved if all the patients had been screened for the blood sugar level, detailed clinical history would have been taken and follow up visits of the patients would have been done in the study.
Direct microscopic examinations of the Gram’s stained smear of high vaginal swabs revealed budding yeast cell only in 7 (14%) cases and all of these cases yielded Candida on culturing. It again re-emphasizes the fact that culture is more sensitive than microscopy [21]. Majority of high vaginal swabs – 40 (80%) revealed few pus cells i.e., < 5 pus cells/ HPF. A study from Sulaiman SP et al., observed that funguria was not associated with the pus cells [22]. Here VVC was not associated with presence of vaginal pus cells.
There was only one causative agent of VVC in 98% of cases in the study as reported by other studies [13,14,23,24]. One mixed infection of C. glabrata and C. albicans was identified on Candida CHROMagar. It was observed in 2% of positive culture and 0.5% of VVC cases. Fan SR et al., identified the mixed causative agent in 2.2% of cases which is similar to the current study while Richter SS et al., identified the mixed causative agent in 14.9% of the vaginal samples by using chromogenic culture medium [25,26]. Incorporation of Candida CHROMagar in the culture medium helps in the detection of mixed infection. Twenty high vaginal swabs (40%) gave a colony count ≥ 105 colonies/ml. High colony count help in differentiating between causative pathogen and colonizers.
NAC (82.4%) were isolated more than C. albicans (17.6%) in the study. C. glabrata (45.1%) was the most common species isolated, followed by C.tropicalis (23.5%), C. albicans (17.6%), C. krusei (9.8%) and C. parapsilosis (3.9%). Similar to our results, various studies have reported C. glabrata as the most common pathogen isolated from VVC [14,27]. A study from Goswami R et al. observed C. glabrata as the most common species isolated from diabetes mellitus patients and its frequency was higher when compared to control group [6]. In contrast to our study, a number of studies identified C. albicans as the most common causative agent; 85.2% in Argentina [23], 73.9% in Kuwait [24], 67% in Iran [28], 89% in Australia [29] and 35.5-66% in India [13,30]. C. glabrata in India varied widely from the most common isolate (61.3%) to less than 2% [13,14,31]. The current study is among the few study from India which depicted isolation of C. glabrata as more than 50% of VVC. Mohanty S et al. and Ray D et al., from AIIMS, New Delhi, India, observed 50.4% and 61.3% VVC by C. glabrata respectively [14,31]. The high isolation of C. glabrata in our hospital may be due to the fact that complicated cases, chronic cases or unresponsive cases of VVC may be presented at this tertiary care hospital. AIIMS, New Delhi, India is also one of the tertiary care hospitals as our hospital. Therefore our finding is in correlation with the finding from AIIMS. In several studies C. glabrata was the second most common causative agent of VVC after C. albicans [32]. Similar to our study Vijaya D et al., reported 26.4% C. tropicalis and 3.8% C. krusei [13].
Antifungal susceptibility testing of Candida species plays a vital role to establish the susceptibility patterns of isolates recovered from different regions in order to guide empirical therapy. Similar to our study, several studies have been reported on fluconazole resistance in C. glabrata [33,34]. Mohanty S et al., performed AFST of representative vaginal isolates and observed similar observation that 30% of Candida spp. were S-DD while none of the Candida spp. were found resistant to fluconazole [14]. Raghunathan L et al., recently studied AFST of 40 vaginal Candida isolates at Puducherry by E-test method and observed that 87.5% were susceptible to fluconazole, 7.5% were S-DD and only 5% were resistant [18]. The increased resistant against fluconazole in the current study was mainly because of high isolation of C. glabrata and C. krusei strains which have increased resistance against fluconazole. Hence, it is recommended to have laboratory based treatment of VVC at tertiary care hospital rather than syndromic management. It will prevent development of resistant cases in the population. It was also observed that all strains of C. albicans were found to be fluconazole susceptible. The VVC infections caused by fluconazole resistant C. albicans have been rare [26]. Hence, it is important to know at least the causative agent as C. albicans or NAC so that accordingly fluconazole based regimen or non-fluconazole based azole regimen respectively can be provided [8,26]. Vaginal isolates from patients with recurrent VVC remain susceptible to fluconazole but may show increased resistance if the patient is on long-term exposure [35]. Further studies are is required to evaluate whether fluconazole resistance was due to infection of primary resistant strains of Candida spp. or acquired resistance due to long term repeated exposure of fluconazole among recurrent vulvovaginal candidiasis.
It is one of the few studies from Southern part of India which is describing voriconazole susceptibility of VVC isolates. None of the Candida species were found to be resistant to voriconazole. Similar to our results, Nguyen MH and Christine Yu CY [36] showed voriconazole in vitro activity against fluconazole-susceptible or fluconazole-resistant Candida isolates. Marcos-Arias C et al., observed that voriconazole as a useful alternative for recurrent oral candidiasis [37]. Ying C et al., observed only 81% of C. albicans strains were susceptible to voriconazole in three maternity hospitals in Shanghai [10]. Vijaya D et al., also observed all strains of C. parapsilosis and C. glabrata, 91.4% of C. albicans, 85.7% of C. tropicalis and 50% of C. krusei were susceptible to voriconazole [13]. Hence, continuous monitoring of antifungal susceptibility testing should be done to guide empirical treatment and in vitro susceptibility testing should be required for NAC isolates to guide appropriate treatment of VVC.
Conclusion
The study strongly recommends laboratory based diagnosis and in vitro susceptibility testing specifically for NAC isolates in a tertiary care hospital. Chromogenic medium facilitate the detection of mixed fungal infection and may be included especially among recurrent vulvovaginal candidiasis. Antifungal susceptibility testing plays an important role in the treatment of VVC because recurrent vulvovaginal candidiasis is difficult to treat.
[1]. Genital / vulvovaginal candidiasis (VVC) | Fungal Diseases | CDC [Internet]. [cited 2016 Jun 20]. Available from: https://www.cdc.gov/fungal/diseases/candidiasis/genital/ [Google Scholar]
[2]. Linhares LM, Witkin SS, Miranda SD, Fonseca AM, Pinotti JA, Ledger WJ, Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by cultureInfect Dis Obstet Gynecol 2001 9(4):221-25. [Google Scholar]
[3]. Sobel JD, Candidal vulvovaginitisClin Obstet Gynecol 1993 36(1):153-65. [Google Scholar]
[4]. Geiger AM, Foxman B, Risk factors for vulvovaginal candidiasis: A case-control study among university studentsEpidemiol Camb Mass 1996 7(2):182-87. [Google Scholar]
[5]. De Leon EM, Jacober SJ, Sobel JD, Foxman B, Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetesBMC Infect Dis 2002 2:1 [Google Scholar]
[6]. Goswami R, Dadhwal V, Tejaswi S, Datta K, Paul A, Haricharan RN, Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic statusJ Infect 2000 41(2):162-66. [Google Scholar]
[7]. Rathod SD, Buffler PA, Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documentedBMC Womens Health 2014 14(1):43 [Google Scholar]
[8]. Vulvovaginal Candidiasis - 2015 STD Treatment Guidelines [Internet]. [cited 2016 Jun 20]. Available from: http://www.cdc.gov/std/tg2015/candidiasis.htm [Google Scholar]
[9]. Yera H, Poulain D, Lefebvre A, Camus D, Sendid B, Polymicrobial candidaemia revealed by peripheral blood smear and chromogenic mediumJ Clin Pathol 2004 57(2):196-98. [Google Scholar]
[10]. Ying C, Zhang H, Tang Z, Chen H, Gao J, Yue C, Antifungal susceptibility and molecular typing of 115 Candida albicans isolates obtained from vulvovaginal candidiasis patients in 3 Shanghai maternity hospitalsMed Mycol 2016 1;54(4):394-99. [Google Scholar]
[11]. Jindal N, Gill P, Aggarwal A, An epidemiological study of vulvovaginal candidiasis in women of childbearing ageIndian J Med Microbiol 2007 25(2):175-76. [Google Scholar]
[12]. Shrivastav V K, Shukla D, Shrivastav A, Jana AM, Prevalence of vaginal candidiasis in diabetic women of Madhya Pradesh, IndiaInt J Curr Microbiol App Sci 2015 4(5):834-46. [Google Scholar]
[13]. Vijaya D, Dhanalakshmi TA, Kulkarni S, Changing trends of vulvovaginal candidiasisJ Lab Physicians 2014 6(1):28-30. [Google Scholar]
[14]. Mohanty S, Xess I, Hasan F, Kapil A, Mittal S, Tolosa JE, Prevalence and susceptibility to fluconazole of Candida species causing vulvovaginitisIndian J Med Res 2007 126(3):216-19. [Google Scholar]
[15]. Baradkar VP, Mathur M, Kumar S, Hichrom candida agar for identification of Candida speciesIndian J Pathol Microbiol 2010 53(1):93-95. [Google Scholar]
[16]. Amar CS, Ashish J, Vinay Hajare, Sreekantha Yogesh B, Vinodchandran Study of prevalence and antifungal susceptibility of candidaInt J Pharma Bio Sci 2013 4(2):361-81. [Google Scholar]
[17]. CLSI. Methods for antifungal disc diffusion susceptibility testing of yeasts; Approved Guideline-Second edition. CLSI document M44-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2009 [Google Scholar]
[18]. Ragunathan L, Poongothai GK, Sinazer AR, Kannaiyan K, Gurumurthy H, Jaget N, Phenotypic characterization and antifungal susceptibility pattern to fluconazole in Candida species isolated from vulvovaginal candidiasis in a tertiary care hospitalJ Clin Diagn Res 2014 8(5):DC01-04. [Google Scholar]
[19]. Ahmad A, Khan AU, Prevalence of Candida species and potential risk factors for vulvovaginal candidiasis in Aligarh, IndiaEur J Obstet Gynecol Reprod Biol 2009 144(1):68-71. [Google Scholar]
[20]. Nandan D, Gupta YP, Krishnan V, Sharma A, Misra SK, Reproductive tract infection in women of reproductive age group in Sitapur/Shahjahanpur district of Uttar PradeshIndian J Public Health 2001 45(1):8-13. [Google Scholar]
[21]. Marot-Leblond A, Nail-Billaud S, Pilon F, Beucher B, Poulain D, Robert R, Efficient diagnosis of vulvovaginal candidiasis by use of a new rapid immunochromatography testJ Clin Microbiol 2009 47(12):3821-25. [Google Scholar]
[22]. Sulaiman SP, Singh R, Mandal J, Fungal profile of funguria cases at a tertiary care hospital in Southern IndiaIndian J Med Res 2014 140(4):556-59. [Google Scholar]
[23]. Gamarra S, Morano S, Dudiuk C, Mancilla E, Nardin ME, de Los Angeles Méndez E, Epidemiology and antifungal susceptibilities of yeasts causing vulvovaginitis in a teaching hospitalMycopathologia 2014 178(3-4):251-58. [Google Scholar]
[24]. Alfouzan W, Dhar R, Ashkanani H, Gupta M, Rachel C, Khan ZU, Species spectrum and antifungal susceptibility profile of vaginal isolates of Candida in KuwaitJ Mycol Médicale 2015 25(1):23-28. [Google Scholar]
[25]. Fan SR, Liu XP, Li JW, Clinical characteristics of vulvovaginal candidiasis and antifungal susceptibilities of Candida species isolates among patients in Southern China from 2003 to 2006J Obstet Gynaecol Res 2008 34(4):561-66. [Google Scholar]
[26]. Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA, Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent casesJ Clin Microbiol 2005 43(5):2155-62. [Google Scholar]
[27]. Kumar CPG, Menon T, Biofilm production by clinical isolates of Candida speciesMed Mycol 2006 44(1):99-101. [Google Scholar]
[28]. Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M, The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient populationEur J Obstet Gynecol Reprod Biol 2011 155(2):199-203. [Google Scholar]
[29]. Holland J, Young ML, Lee O, C-A Chen S, Vulvovaginal carriage of yeasts other than CandidaalbicansSex Transm Infect 2003 79(3):249-50. [Google Scholar]
[30]. Babin D, Kotigadde S, Rao P, Rao TV, Clinico-mycological profile of vaginal candidiasis in a tertiary care hospital in KeralaInt J Res Biol Sci 2013 3(1):55-59. [Google Scholar]
[31]. Ray D, Goswami R, Banerjee U, Dadhwal V, Goswami D, Mandal P, Prevalence of Candidaglabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasisDiabetes Care 2007 30(2):312-17. [Google Scholar]
[32]. Pirotta MV, Garland SM, Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibioticsJ Clin Microbiol 2006 44(9):3213-17. [Google Scholar]
[33]. Bennett JE, Izumikawa K, Marr KA, Mechanism of increased fluconazole resistance in Candidaglabrata during prophylaxisAntimicrob Agents Chemother 2004 48(5):1773-77. [Google Scholar]
[34]. Posteraro B, Tumbarello M, La Sorda M, Spanu T, Trecarichi EM, De Bernardis F, Azole resistance of Candidaglabrata in a case of recurrent fungemiaJ Clin Microbiol 2006 44(8):3046-47. [Google Scholar]
[35]. Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD, Fluconazole-resistant Candidaalbicans vulvovaginitisObstet Gynecol 2012 120(6):1407-14. [Google Scholar]
[36]. Nguyen MH, Yu CY, Voriconazole against fluconazole-susceptible and resistant candida isolates: in vitro efficacy compared with that of itraconazole and ketoconazoleJ Antimicrob Chemother 1998 42(2):253-56. [Google Scholar]
[37]. Marcos-Arias C, Eraso E, Madariaga L, Carrillo-Muñoz AJ, Quindós G, In vitro activities of new triazole antifungal agents, posaconazole and voriconazole, against oral Candida isolates from patients suffering from denture stomatitisMycopathologia 2012 173(1):35-46. [Google Scholar]