Root canal instrumentation comprises of the combined action of endodontic instruments and irrigating solutions which is aimed at the elimination of pre-existing organic and inorganic debris resulting from the operative procedures as well as the reduction of the microbial content and it’s by products [1].
Irrigating solutions used during endodontic treatment may lead to alterations in the chemical structure which may in turn affect the mechanical properties of dentin. These irrigants used for removal of smear layer may act similarly on the smear layer as well as the root dentin. It consequently leads to the exposure of collagen and eventually causes decrease in dentin micro-hardness.
Pashley D et al., suggested that an inverse relation exists between the dentin micro-hardness and density of the dentinal tubules. Reduced micro-hardness may lead to reduction in modulus of elasticity and flexural strength of dentin [2]. Hence the determination of micro-hardness provides an arbitrary assessment of the change in any mineral content of dental hard tissues [2].
A 17% Ethylene Dioxide Tetra Acetic Acid (EDTA) has been routinely used as a root canal irrigant for removal of smear layer [3]. More recently 0.2% Chitosan and 6% Morindacitrifolia Juice (MCJ) are being considered for irrigation of root canal due to their antimicrobial properties and efficacy in removal of smear layer [4].
A 0.2% Chitosan which is an animal product is used for smear layer removal. Its mechanism of action is not yet clear, but it is thought that adsorption, ionic exchange and chelation property may be responsible for formation of the complex between substance and metallic ions. There are two theories of chitosan which explains its chelating process. They are:
On comparision to EDTA, the Chitosan polymer consists of a chain of many dimers of chitin. The dimers of chitin have two nitrogen atoms and two free electrons that are liable for the interaction of ions between the chelating agent and the metal. In the acidic medium, protonation of amino acid results in a complete position change (-NH3+) which is responsible for the attraction of other molecules for adsorption to occur [7].
This study was designed to evaluate the effect of various root canal irrigating solutions- 3% NaOCl, 17% EDTA, 0.2% Chitosan and 6% MCJ on the micro-hardness of the root canal dentin by Vickers micro-hardness test.
Materials and Methods
This in vitro study was designed and conducted in the Department of Conservative and Endodontics, College of Dental Science and Hospital Rau Indore, within a period of three months from June to August 2016. Samples size was decided taking into consideration other similar in vitro studies [10].
Eighty freshly extracted non carious premolars were selected for the study and stored in 0.1% thymol solution. Non carious, non fractured, non restored, single rooted premolars were included in the study and the carious, fractured, restored and multi rooted teeth were excluded from the study. The selected samples were decoronated at the level of cemento-enamel junction with the help of a water cooled diamond impregnated disc. No endodontic treatment was initiated for the samples. For longitudinal sectioning, grooves were created on the buccal and lingual external surface of roots without penetrating into the canals using a double faced diamond disc under water cooling, and with the help of chisel, the roots were split into two halves [Table/Fig-1].
Showing longitudinal sectioning of selected samples.
The sectioned specimens were then examined under a stereo-microscope (Model TZ-240) to identify teeth with cracks. No teeth were found to have cracks; hence all the eighty samples were included in the study. All the samples were then ground polished with water cooled carborandum disc and finally polished with felt disc followed by buffing using 0.05 μm sized aluminium oxide powder mixed with distilled water [1].
Freshly mixed auto polymerized resin was poured in plastic rings of uniform diameter. The specimens were embedded on the resin with the polished surface facing outwards. After curing of resin, the rings were removed and re-polishing of specimens was performed to remove excess material present on the tooth surface [Table/Fig-2].
All the samples were individually mounted on the stage of Vicker’s micro-hardness tester and indentations were marked with a Vicker’s diamond indenter at 300 gm load and dwell time of 20 seconds for measuring baseline data.
Commercially available solutions of 3% NaOCl {Vishal laboratory PVT., LTD (India)} and 17% EDTA {Prime Dent Products PVT., LTD (India)} were used. For the study 0.2% Chitosan solution was prepared by mixing 100 ml of acetic acid with 0.2 g of Chitosan powder [4] {i-CHESS Chemicals Pvt., Ltd. (Bandra West Mumbai)}. The mixture was then stirred for 24 hours using a magnetic stirrer. Similarly, 6% MCJ was freshly prepared by diluting 6 ml of MCJ {Kisalaya Herbal Ltd (Indore)} with 100 ml of normal saline using a pipette.
All the four irrigating solutions were subjected to pH test, which was done using a digital pH meter and the pH readings were recorded.
After obtaining the baseline Vicker’s Hardness Number (VHN) results of all the eighty specimens, they were divided into groups of twenty samples each. The specimens in these groups were then immersed in the respective irrigating solutions (3% NaOCl, 17% EDTA, 0.2% Chitosan and 6% MCJ) for 15 minutes.
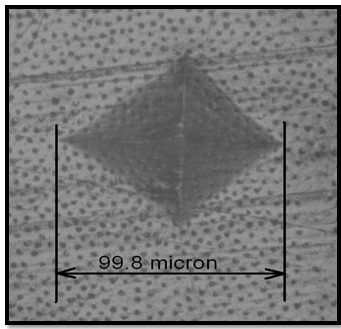
At the end of the active treatment period of 15 minutes, the samples were rinsed with distilled water and dried. The samples were again mounted on the stage of Vicker’s micro hardness tester [Table/Fig-3] and for each sample indentations were marked at three different locations with a Vicker’s diamond indenter at 300 gm load and a dwell time of 20 seconds.
Vicker’s micro hardness tester machine;

All the indentations were marked in the middle region of the roots, approximately half way between the center of the canal lumen and the peripheral cementum where the dentin surface was uniform.
These indentations were measured and converted into VHN values [Table/Fig-4] by the computerized monitor (Model-Leitz-Miniload) [Table/Fig-3]. At the end of 15 minutes of active treatment the irrigating solutions were again subjected to pH test to record any change in pH caused due to the dissolution and resulting buffering action of dentin of the immersed specimens.
Indentation marked in the mid-root region.
Statistical analysis was carried out using one-way ANOVA (for intergroup comparison) along with Post-hoc Tukey test (to find out the pair-wise mean hardness difference between two groups) and Paired t-test (to compare the Mean micro-hardness and mean pH before and after immersion of the samples in their respective irrigants).
Results
The mean hardness among the four groups was compared using the one-way ANOVA. The p-value of > 0.05 was obtained, which is statistically not significant. Therefore, the mean hardness of teeth in all the four groups prior to immersion in irrigants was comparable [Table/Fig-5].
Comparison of Mean Hardness among the four groups before the teeth was immersed in their respective irrigants using one-way ANOVA test.
| (N=80) | 3% NaOCl(n=20) | 17% EDTA(n=20) | 6% Morinda Citrifolia Juice(n=20) | 0.2% Chitosan(n=20) | F Value | p-value |
|---|
| Mean±SD | 57.15±1.75 | 56.88±1.38 | 57.92±1.78 | 57.87±1.60 | 2.030 | 0.117, NS |
The p-value for all the pairs was found to be >0.05, i.e., there was no significant difference found in the mean hardness between any two groups before immersion [Table/Fig-6].
Pair-wise comparison of hardness between the two groups using Post-hoc Tukey test before immersion of samples in their respective irrigant.
| Pairs | p-value | Significance |
|---|
| 3% NaOCl – 17% EDTA | 0.949 | Not significant |
| 3% NaOCl – 6% Morinda Citrifolia Juice | 0.455 | Not significant |
| 3% NaOCl – 0.2% Chitosan | 0.514 | Not significant |
| 17% EDTA – 6% Morinda Citrifolia Juice | 0.190 | Not significant |
| 17% EDTA – 0.2% Chitosan | 0.227 | Not significant |
| 6% Morinda Citrifolia Juice - 0.2% Chitosan | 1.000 | Not significant |
The p-value of < 0.05 was obtained, which was statistically significant. There was a statistically significant difference in the mean hardness among the four groups after the teeth were immersed in their respective irrigants. (6% MCJ > 3% NaOCl> 0.2% Chitosan > 17% EDTA) [Table/Fig-7].
Comparison of Mean Micro- Hardness among the four groups after the teeth were immersed in their respective irrigants using one-way ANOVA test.
| (N=80) | 3% NaOCl(n=20) | 17% EDTA(n=20) | 6% Morinda Citrifolia Juice(n=20) | 0.2% Chitosan(n=20) | F Value | p-value |
|---|
| Mean±SD | 55.15±1.86 | 43.12±2.51 | 56.91±2.11 | 44.65±3.19 | 163.802 | <0.001 |
The pairs 3% NaOCl – 6% Morinda Citrifolia Juice and 17% EDTA – 0.2% Chitosan showed a p-value of > 0.05, which is statistically not significant. Thus, there was no difference in the mean hardness after the treatment between these two pairs of group.
A 3% NaOCl – 17% EDTA, 3% NaOCl – 0.2% Chitosan, 17% EDTA – 6% Morinda Citrifolia Juice and 6% Morinda Citrifolia Juice - 0.2% Chitosan, these pairs showed a p-value of < 0.05, which is statistically significant. Thus, there was a statistically significant difference in these pairs of groups [Table/Fig-8].
Shows the pair-wise comparison of hardness between the two groups using Post-hoc Turkey test after immersion of samples in their respective irrigant
| Pairs | p-value | Significance |
|---|
| 3% NaOCl – 17% EDTA | < 0.001 | Significant |
| 3% NaOCl – 6% Morinda Citrifolia Juice | 0.119 | Not significant |
| 3% NaOCl – 0.2% Chitosan | < 0.001 | Significant |
| 17% EDTA – 6% Morinda Citrifolia Juice | < 0.001 | Significant |
| 17% EDTA – 0.2% Chitosan | 0.213 | Not significant |
| 6% Morinda Citrifolia Juice - 0.2% Chitosan | < 0.001 | Significant |
Immersion of specimen in 6% Morinda citrifolia juice group and 3% NaOCl showed no significant difference (p>0.05) in the microhardness of dentin. However statistically significant difference in the mean hardness was observed in the other two groups (p<0.05) [Table/Fig-9].
Comparison of Mean Micro- Hardness among the Four Groups before and after the teeth was immersed in their respective irrigants using Paired t-test (N=80).
| Group | Before Treatment(Mean±SD) | After Treatment(Mean±SD) | t-value | p-value |
|---|
| 3% NaOCl | 57.15 ± 1.75 | 55.15 ± 1.86 | 0.6844, df=19 | 0.501986, NS |
| 17% EDTA | 56.88 ± 1.38 | 43.12 ± 2.51 | 22.532, df=19 | <0.001 |
| 6% Morinda Citrifolia Juice | 57.92 ± 1.78 | 56.91 ± 2.11 | 1.926, df=19 | 0.069, NS |
| 0.2% Chitosan | 57.87 ± 1.60 | 44.65 ± 3.19 | 15.619, df=19 | <0.001 |
Thus, it may be concluded that in 17% EDTA and 0.2% Chitosan groups, there was a statistically significant reduction in the mean hardness of teeth after treatment, while no change was seen in the 6% Morinda citrifolia juice group and 3% NaOCl.
Significant difference in the pH was observed in all the irrigating solution after immersion of tooth samples for 15 minutes each [Table/Fig-10].
Comparison of Mean pH before and after immersion of the samples in their respective irrigants using Paired t-test
| Group | Before TreatmentpH (Mean±SD) | After TreatmentPh (Mean±SD) | t-value | p-value |
|---|
| 3% NaOCl | 10.6400 ± 0.13514 | 10.0950 ± 0.07237 | 14.128 | <0.001 |
| 17% EDTA | 9.9625 ± 0.21531 | 9.3730 ± 0.27972 | 13.491 | <0.001 |
| 6% Morinda Citrifolia Juice | 4.4860 ± 0.17825 | 4.6000 ± 0.16199 | -2.876 | .010 |
| 0.2% Chitosan | 3.4085 ± 0.10644 | 3.3605 ± 0.09589 | 9.590 | <0.001 |
Discussion
Long term prognosis of root canal treatment is entirely dependent on the quality of instrumentation, irrigation, disinfection and finally the obturation of the root canal system.
During irrigation the coronal as well as radicular dentin is subjected to the action of irrigating solutions which may lead to alteration in the physical and chemical properties of root canal dentin including hardness. Therefore, the aim of present study was to evaluate the effect of various endodontic irrigating solutions i.e., 3% NaOCl, 17% EDTA, 6% MCJ and 0.2% Chitosan on the micro-hardness of root canal dentin.
Micro-hardness testing, is widely used to study fine scale changes in the hardness, either intentional or accidental and is one of the most uncomplicated and non-destructive methods. The micro-hardness of dentin can be measured by Vicker’s hardness test (for deep dentin). Vicker’s hardness test was used as it is suitable and a practical method to evaluate the change in the surface in deeper hard tissue structures. This test is widely accepted because of its extremely accurate readings and the fact that in this method, just one type of indentation is used for all types of surface treatment [11].
Previous investigations concluded that there is a decrease in the micro-hardness when tested from the superficial to deep dentin. This can be, because of the fact that the number of dentinal tubules increases towards the pulp, which provides minimal resistance to dentinal micro-hardness testing indenter [12]. Pashley D et al., compared the microhardness change and concluded that an inverse correlation existed between micro-hardness of dentin and tubular density [2]. The degree of mineralization and hydroxyapatite in the intertubular substance are also determinants of microhardness of dentin [13].
In the present study, the mid root dentin region was selected in order to minimize structural variations and also to obtain uniform baseline results for evaluation. Ground polishing of the samples were done to eliminate any surface irregularities and to obtain a mirror like finish [1]. The glossy surface ensures the reflection of light so that indentations can be clearly visualized when testing with the VHN testing machine.
The time period of 15 minutes for the immersion of the samples was kept constant to maintain uniformity between the four groups [1].
On immersing the samples into the irrigating solutions, it was observed that 6% MCJ had no effect on the micro-hardness of dentin, which was in accordance with the results obtained in the study conducted by Prabhakar AR et al., [10].
In the present study, 3% NaOCl was found to slightly reduce the dentin micro-hardness, although the results were non-significant. The reduction in the dentinal microhardness may be attributed to the depletion of the organic phase (type I collagen) of dentin [14] caused by sodium hypochlorite.
A 17% EDTA demonstrated a significant reduction in micro-hardness of dentin. EDTA favors removal of smear layer by affecting the inorganic content of root canal walls. The fact that 17% EDTA reduces the micro-hardness of dentin could be due to its chelating property. Hulsmann M et al., using gravimetrical analysis reviewed the mode of action of EDTA and observed that the effect of EDTA is self limiting [15]. At a neutral pH (7.4), it showed chemically two co-existing reactions. They are complex formation and protonation [16]. EDTA HNa3 is normal available form of EDTA and the reaction is as follows:
EDTA H3- + Ca2+--> EDTACa2- + H
EDTA H3- + H --> EDTAH22-
The decrease in the pH is due to the removal of calcium ions from dentin by hydrogen ions. Because of the presence of acid, as the time increases its efficiency decreases. On the other hand, the solubility of the dentin is affected by the reaction of acid with hydroxyapatite [16].
For standardization, all samples were immersed in the selected irrigating solutions for 15 minutes [1,17]. The results obtained revealed a significant reduction in dentin micro-hardness on immersion in 17% EDTA for the stipulated time period. Sayin TC et al., also concluded in a study that EDTA used either alone or in combination with NaOCl significantly reduces the micro-hardness of dentin [18].
A 0.2% Chitosan, which is is a chelating agent, was also seen to affect the microhardness of dentin negatively. Previously it was believed that reduction in microhardness of dentin after use of 0.2% Chitosan solution is due to acetic acid present in it [19]. However, studies have revealed that 5% acetic acid does not hamper the microhardness of root canal dentin [20,21]. Thus it may be concluded that the effect of Chitosan in reduction of the microhardness of dentin, may be attributed to the substance and not the acid. Pimenta JA et al., reported that 0.2% Chitosan solution having a pH 3.2 reduces the microhardness of dentin similar to 15% EDTA (pH 7.25) [19].
In the present study 6% MCJ which has been found to be effective in smear layer removal was used in order to evaluate its effect on micro-hardness of dentin. It contains bioactive compounds which are responsible for its antibacterial activity. In addition it contains some organic acids such as caproic acid, ursolic acid and caprylic acid [9,22]. The minor smear layer removal property of MCJ could be due to presence of these acids [4] which could also alter the micro structure of dentin that may be responsible for reduction in micro-hardness of dentin [23]. A 6% MCJ showed non-significant reduction in the micro-hardness of root canal dentin; however the minor reduction in the micro-hardness may have been evident due to the acidic component of MCJ.
Limitation
One of the limitation of the present study is that, it was an in vitro study that evaluated only the microhardness of the dentin; and further clinical trials are needed for establishing the effectiveness, safety and biocompatibility of herbal irrigants before they can be used routinely in vivo.
Conclusion
Within the limitations of this in vitro study, it may be concluded that all the used irrigating solutions affected the micro-hardness of root canal dentin.
In the present study it was found that the results obtained with the use of 6% MCJ and 3% NaOCl does not significantly affect the dentin micro-hardness in contrast to the other two irrigants used i.e., 17 % EDTA and 0.2% Chitosan.
Hence, it may be concluded that herbal irrigants like MCJ may serve as an effective alternative to the conventionally used root canal irrigants as they cause minimal alteration of dentin structure in addition to being less toxic when compared with synthetic irrigants.