PE is a major cause of maternal and neonatal morbidity and mortality. It is a multisystem disorder which is characterized by vasoconstriction [1], leukocyte activation [2], enhanced inflammatory response [3] and oxidative stress [4]. The causes for the development of PE are still unclear and are a topic of active investigation. The pathological lesions of decidual vessels in PE have similarity to atherotic lesions of arteries [5]. Neutrophils have been implicated in the pathogenesis of atherotic changes and endothelial dysfunction through release of variety of substances. Elastase is one of such molecules released from neutrophils and is an established marker for neutrophil activation [6–8].
Neutrophil Elastase (NE), a serine protease stored in the primary granules of neutrophils, is capable of degrading various extracellular matrix proteins such as elastin, collagen, fibrinogen and proteoglycans [9]. Therefore, it can cause vascular basement membrane damage and can facilitate tissue infiltration of neutrophils. Activation of neutrophils is implicated in PE and consequently contributes to vascular basement membrane damage leading to oedema and proteinuria [10], a usual observation in PE. A positive correlation have been demonstrated between Von Willebrand Factor (a marker of endothelial damage) and NE by Greer IA et al., indicating that neutrophil activation could contribute to endothelial damage and dysfunction in PE [11].
Thus, uncontrolled neutrophil activation can lead to destruction of the integrity of endothelial cells and could exacerbate the pathophysiological symptoms in PE. It is well established that PE is manifested as mild, moderate and severe forms in pregnant women but it is unclear what exaggerates the symptoms and the severity. This study is an attempt in this direction to correlate the activity of neutrophil elastase and its endogenous inhibitors α1-antitrypsin (α1-AT) and α2-macroglobulin (α2-MG) with severity of PE.
Materials and Methods
The present study is a comparative study conducted during the period of October 2015 to April 2016. The subjects of this study were the pregnant women attending or admitted in the Department of Obstetrics and Gynecology, RL Jalappa Hospital and Research Center, the teaching hospital of Sri Devaraj Urs Medical College (SDUMC) and the biochemical evaluation was carried out in the Department of Biochemistry of SDUMC, a constituent college of Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India. Every enrolled pregnant woman gave their informed written consent to participate in the study. This study was performed after obtaining Institutional Ethical Committee approval and the study complied with the Helsinki Declaration.
A total of 50 pregnant normotensive women and 50 pre-eclamptic pregnant women (27 mild and 23 severe cases), were included in the study. All the women were in the age group of 19-36 years and were over 20 weeks of gestation. Normal pregnancy was diagnosed on the basis of clinical and ultrasound evaluation and all of them presented a normal course and outcome of pregnancy. The pre-eclamptic patients were diagnosed by the presence of hypertension (≥140 mmHg systolic BP and ≥90 mmHg diastolic BP) on two occasions with 4-6 hours apart, proteinuria (≥1+ by urine dipstick method) with or without pathological oedema. PE was considered as severe, if the subjects had at least two of the following: ≥160 mmHg systolic BP; ≥110 mmHg diastolic BP; dipstick proteinuria of 3+ or more. All the other cases were considered as mild PE. All patients with any infection, twins, history of pregestational diabetes, gestational diabetes mellitus, renal disease, liver disease, cardiovascular disease and hypertension were excluded from the study.
Almost 6 ml of blood was collected from an antecubital vein from all the subjects in tubes containing EDTA (for haematologic studies); Sodium heparin (for NE, α1-AT, α2-MG and NE- α1-ATcomplex estimation) and in tubes without anticoagulant (for CRP estimation). Blood samples were centrifuged within two hours of collection. After centrifugation, serum and plasma were separated and aliquots were stored at -70oC until assayed. Samples were thawed at room temperature, vortexed and centrifuged before analysis.
Complete blood count was performed by Beckman- Coulter, an automatic blood cell counter. Serum C - Reactive Protein (CRP) estimation was done by rapid latex slide tests. Serum uric acid was estimated by uricase method [12] using Dry Chemistry Vitros 250 Johnson and Johnson analyzer. Estimation of plasma elastase was done using succinyl tri- L-alanyl-p-nitroanilide (STANA, from SIGMA) as substrate at 410 nm as per the procedure described by Beith J et al., [13]. Plasma α1-AT and α2-MG were analyzed using ELISA kit purchased from Immunology Consultants laboratory, Inc, USA. NE-α1-AT complex was quantified by ELISA (Calbiochem).
Statistical Analysis
The data were statistically analyzed by SPSS software version 22.0. The results were expressed as mean±SD. For statistical differences in means between the groups ANOVA (Analysis of variance) was used. Pearson’s correlation coefficient test was used to analyze the correlation of severity parameters (BP and proteinuria) with elastase and its inhibitors. A p-value <0.001 was considered highly significant.
Results
The baseline physical and biochemical characteristics of the normal, mild and severe pre-eclamptic subjects are depicted in [Table/Fig-1]. The gestational age was in the range of 34 to 37 weeks for normal, and 31 to 36 weeks for mild to severe PE subjects. The blood pressure was elevated significantly in the case of mild and severe cases of PE in comparison to normal. The blood pressure was also significantly higher in severe PE compared to mild PE. The data on proteinuria was suggestive of PE as per the criteria defined. Serum uric acid showed significant rise in PE group (mild 7.53±1.35; severe 8.16±1.57) compared to controls (4.53±1.30). When serum CRP was compared, mild (12.44±11.40) and severe (14.35±13.98) pre-eclamptic women presented significantly higher CRP levels as compared to normotensive pregnant women.
Baseline characteristics of study groups.
| Variables | Normal pregnancy (n=50) | Mild pre-eclampsia (n=27) | Severe pre-eclampsia (n=23) | p-value |
|---|
| Maternal age (years) | 23.62±2.98 | 25.11±4.20 | 25.70±4.35 | |
| Gestational age (weeks) | 37.18±3.05 | 34.70±3.37 | 34.96±3.11 | |
| Blood Pressure (mmHg) | | | | |
| Systolic | 120.28±8.70 | 147.11±9.35 | 170.87±14.11 | *p<0.001 |
| Diastolic | 80.20±5.88 | 100.22±8.84 | 106.52±11.12 | |
| Cases with proteinuria n (%) | - | | | |
| • Traces | | 4 (14.8) | 1(4.3) | |
| • 1+ | | 16 (59.3) | 3(13.0) | |
| • 2+ | | 7 (25.9) | 5(21.7) | |
| • 3+ | | - | 14(60.9) | |
| Serum uric acid (mg/dl) | 4.53±1.30 | 7.53±1.35 | 8.16±1.57 | *p<0.001 |
| Serum CRP (ug/ml) | 0 | 12.44±11.40 | 14.35±13.98 | *p<0.001 |
ANOVA with Bonferroni test was used. Results represented as mean±SD.*p<0.001 statistically highly significant.
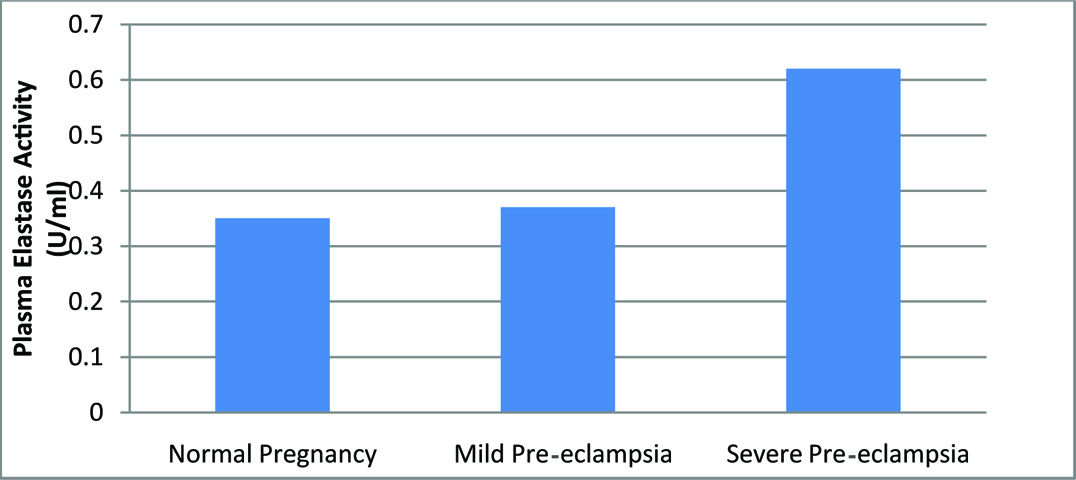
The data on NE, α1-AT, α2-MG and NE- α1-AT complex are presented in [Table/Fig-2]. The activity of neutrophil elastase was increased two fold in severe PE (0.62±0.08) in comparison to normal (0.35+0.10) and mild preeclamptic subjects (0.37±0.03) and was statistically highly significant. The values of α1-AT have been on the decline and were significantly less in mild and severe PE in comparison to normal; a 60% reduction in severe and 40% reduction in mild. There was a significant rise in the levels of α2-MG in severe pre-eclamptic women. However, the complex estimation did not evince any significant changes indicating normal balance and did not contribute to analytic value. Significant association between elastase activity and disease severity is depicted in [Table/Fig-3].
Plasma levels of elastase activity, α1-AT, α2-MG and NE-α1-AT complex in the study groups.
| Parameters | Normal pregnancy (n=50) | Mild pre-eclampsia (n=27) | Severe pre-eclampsia (n=23) | p-value |
|---|
| Plasma Elastase Activity (U/ml) | 0.35±0.10 | 0.37±0.03 | 0.62±0.08 | *p<0.001 |
| Plasma α1-AT (mg/dl) | 110.26±42.39 | 83.94±25.08 | 68.58±26.39 | *p<0.001 |
| Plasma α2-MG (mg/dl) | 265.37±66.91 | 201.06±38.23 | 298.79±32.52 | *p<0.001 |
| Plasma NE-α1-ATcomplex (ng/ml) | 171.08±23.81 | 176.19±9.27 | 164.31±11.63 | p=0.285 |
ANOVA with Bonferroni test was used. Results represented as mean±SD. *p<0.001 statistically highly significant.
Elastase activity expressed in U/ml of plasma in study groups.
Correlation studies of the severity parameter; proteinuria with the levels of NE and α1-AT indicated a positive and negative picture respectively in severe form of PE [Table/Fig-4]. On the other hand, the correlation analysis of α2-MG with proteinuria presented a negative correlation in mild and a positive correlation in severe group. However, complex correlation with the severity parameters did not present any definite correlations.
Correlation of plasma levels of elastase activity, α1-AT, α2-MG and NE-α1- AT complex with severity markers in mild and severe groups.
Pearson’s correlation coefficient test was used.
| Parameters | Plasma Elastase Activity (U/ml) | Plasma α1-ATAT (mg/dl) | Plasma α2-MG (mg/dl) | Plasma NE -α1-ATATcomplex (ng/ml) |
|---|
| r | P | r | P | r | P | r | P |
|---|
| Mild PE |
| Systolic BP (mmHg) | -0.178 | 0.373 | 0.361 | 0.064 | 0.131 | 0.156 | -0.130 | 0.673 |
| Diastolic BP (mmHg) | -0.004 | 0.983 | -0.201 | 0.314 | 0.005 | 0.978 | 0.280 | 0.354 |
| Proteinuria -traces | 0.899 | 0.101 | -0.690 | 0.310 | -0.856 | 0.144 | 1.000 | - |
| Proteinuria -1+ | 0.014 | 0.960 | 0.266 | 0.338 | -0.436 | 0.104 | -0.338 | 0.412 |
| Proteinuria -2+ | 0.090 | 0.832 | 0.046 | 0.914 | -0.076 | 0.857 | -0.987 | 0.104 |
| Severe PE |
| Systolic BP (mmHg) | -0.015 | 0.945 | -0.075 | 0.733 | -0.263 | 0.224 | -0.258 | 0.419 |
| Diastolic BP (mmHg) | -0.376 | 0.077 | 0.153 | 0.486 | 0.054 | 0.808 | 0.182 | 0.572 |
| Proteinuria -1+ | 0.064 | 0.959 | -0.247 | 0.841 | 0.933 | 0.234 | 0.920 | 0.256 |
| Proteinuria -2+ | 0.366 | 0.544 | -0.282 | 0.645 | 0.692 | 0.195 | -0.331 | 0.586 |
| Proteinuria -3+ | 0.169 | 0.565 | -0.171 | 0.558 | 0.290 | 0.315 | 0.329 | 0.589 |
Discussion
PE exhibits characteristics of an inflammatory disease including neutrophil activation [2,14,15]. The activation of neutrophils in PE may be due to some pro-inflammatory cytokines and chemoattractants released during an inflammatory response (i.e., TNF-α, IL-6 and IL-8) [16]. Elastase activity is measured as marker of neutrophil activation in several inflammatory conditions including PE [7,8,14,15]. The complications induced by PE state are detrimental to both the mother and the foetus and have been a serious subject of investigation. Research often focuses on the changes in the biochemical parameters with no data on its onset and progress.
In the present study we have measured and compared the plasma levels of NE, α1-AT, α2-MG and NE- α1-AT complex in patients suffering from mild and severe PE with normal pregnant women [Table/Fig-2]. The analysis of NE activity indicated a significant increase in the severe PE which is in agreement with previous studies [7,8,11,14] and it could contribute to progressive inflammation.
α1-antitrypsin inhibits several serine proteases (mainly NE), and adequate activity of this inhibitor is critical for the maintenance of protease–antiprotease homeostasis and the prevention against proteolytic tissue damage [17]. Determination of the plasma α1-AT level demonstrates the available level of the inhibitor capable of inhibiting intravascular proteases. Contrary to expectation of an increased level of α1-AT in inflammation, a decreased level was observed in the study groups compared to the normal and it was highly significant paving ways to overpowering role of elastase in the complications of PE.
It is also pertinent to note that, there was no increase in the levels of NE-α1-AT complex in PE group consequent to the increased elastase activity. This observation could be an indication of decreased synthesis of α1-AT rather than its involvement in complex formation to control elastase activity. The reason for decreased α1-AT is an area of concern and is suggestive that supplementation of α1-AT would be able to minimize the destructive effects of NE on vascular tissues.
We have observed a significantly higher α2-MG level in severe PE patients compared with normal or mild PE patients against an expected reduced α2-MG concentration in severe PE as it is supposed to bind to elastase and get rapidly cleared from the plasma through macrophage receptors [18]. Raised levels observed in this study could be attributed to renal insufficiency, a common feature in PE patients. Moreover, increased levels of this inhibitor in severe PE possibly contribute to the intravascular coagulation as α2-MG has antiplasmin activity [19] adding to further severe complications. Horne CHW et al., also found high α2-MG levels in PE with proteinuria as compared to normal pregnant women [19].
In order to understand the relation of PE severity parameters with the levels of NE, α1-AT, α2-MG and NE- α1-AT complex correlation analyses were carried out. Though we have correlated these molecules with both proteinuria and BP, the most meaningful and relevant correlation was observed only with proteinuria and not with BP. The observation of increase in the activity of NE in severe PE and its positive correlation with severity marker proteinuria makes the measurement of NE a dependable parameter in the determination of severity of PE. Similar picture but in a negative direction was observed in case of α1-AT suggesting that measurement of α1-AT along with the activity of NE could strengthen the assessment of severity of PE.
As reported by earlier studies [20–22], a significant increase in the levels of serum uric acid and CRP in PE compared to controls was observed and points to generalised inflammation in these patients. However, the levels did not yield any information on the severity of the PE.
Limitation
Since PE is a progressive disease, a follow up of mild preeclamptic women to assess the progression of these women to severe form would have been the better study design to emphatically conclude the role of elastase enzyme and its inhibitors in severity of PE. Moreover, urinary estimation of α1-AT would have explained the reduced levels of α1-AT in PE group. We do agree a larger sample size definitely would have supported the results obtained.
Conclusion
The present study clearly indicates an association between increased levels of NE and decreased α1-AT with the severity of PE suggesting that both would be relevant markers for assessment of severity. Monitoring the plasma levels of these molecules thus could be of use for evaluation of the status of PE.
ANOVA with Bonferroni test was used. Results represented as mean±SD.*p<0.001 statistically highly significant.
ANOVA with Bonferroni test was used. Results represented as mean±SD. *p<0.001 statistically highly significant.