Introduction
The concept of vascular and biliary segments in human liver, as eight segment scheme formalized by Couinaud in 1957, had gained a worldwide acceptance [1]. The Couinaud segmental anatomy is currently used widely, since it is best suited for surgery and have become essential in monitoring various intra parenchymal lesions. The increasing number of variations encountered during imaging studies and in surgical operations forced a thorough search in recent literature, and proposes new concepts of liver segmentation or even a new liver anatomy [2]. Recent advances in surgical and radiological techniques including reduced liver size for pediatric and adult transplants, split liver, and living donor in liver transplantation, make the re-examination of hepatic vascular segmentations to have rationale international consensus [3]. The classification of portal vein anatomy is the basis for the surgical anatomy of hepatic segments and sub segments. The anatomy of the hepatic veins, which form an outer frame of the hepatic segments, is also important in the delimitation of the segments. A detailed knowledge of portal and venous anatomy as well as its principal variants is essential in the proper systematization of the liver, which allows exact detection of a lesion or in the preparation for surgical intervention. The anatomical variations of the vascular pedicles play a major role in determining the segmentations and the location of liver lesions in more precise manner and may alter the approach to hepatic surgery [4].
The knowledge of vascular variants can have critical implications during surgery and in interventional radiological procedures, such as transplantation, complex hepatobiliary surgical and percutaneous procedures, including trisegmentectomy, Portal Vein Embolization (PVE), Transjugular Intrahepatic Portosystemic Shunts (TIPS), Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) and radioembolization/Selective Internal Radiation Therapy (SIRT) [5]. Therefore, in order to reduce the postoperative complications a brief knowledge of the hepatic vascular as well as biliary anatomic variants is mandatory before planning the surgery. Modern non-invasive diagnostic imaging techniques such as Multi Detector Computed Tomography (MDCT) and Magnetic Resonance Imaging (MRI) are available preoperatively to help the surgeons or interventional radiologists to know the entire liver vasculature and, thereby, help them to adopt the best treatment plan [6]. The present review aims at explaining the variations in the intrahepatic portions of portal venous radicals and to examine the implications of these variants in the segmentations of liver and in various surgical and radiological interventional procedures.
A literature search was performed from Pubmed, Scopus and Google scholar data bases from January 2000 to December 2016. The search terms such as portal variants, liver segmentations, the surgical implications like PVE, TIPS, liver resection and transplantation, ALPPS and radioembolization/SIRT were used. From the titles identified, the full text articles reporting the above search terms were screened by the authors and only the potential relevant studies were included in this review.
Embryological Insight
The portal vein develop during second and third month of gestation from two vitelline veins, which drain the yolk sac. The two vitelline veins form a plexus around the duodenum by forming three bridging anastomosis, two ventral and one dorsal, and cross the septum transversum. The proliferating liver buds break up this network and form liver sinusoids. Beyond the developing liver, the vitelline veins gets transformed into hepatocardiac channels, which due to reorganization of blood circulation become suprahepatic segment of inferior vena cava. The portal vein develops from selective regression of paraduodenal anastomosis, by hemodynamic laws favouring the shortest paths after the rotation of duodenum, and forms a single vessel. The stem of the portal vein is derived from left vitelline vein and its dorsal anastomosis; the left branch develops from both left vitelline vein and cranial ventral anastomosis and the right branch is formed by part of right vitelline vein. Deviations from the normal process of these anastomosis results in the variations in the branching pattern of portal vein. The umbilical veins, which transport oxygenated blood from placenta, slowly merge with the developing liver sinusoids during the development of liver. The right umbilical vein and the hepatic portion of the left umbilical vein disappear. With the increase in the placental circulation, a direct extrahepatic shunt ductus venosus appears between the left umbilical vein and the right hepatocardiac channel and bypasses the sinusoidal plexus of liver. After birth this communication disappears, and the left umbilical vein modifies to form round ligament and venous duct gives rise to venous ligament. All these changes begin by 4th week and completed by 12th week of intrauterine period [7,8].
Portal Variants
The portal vein has a segmental intrahepatic distribution and it closely follows the hepatic artery. Cheng Y et al., classified the intrahepatic portal vein variations into five different types, which was subsequently followed by other observers in describing the variants [9]. After its entry through hilum, the Main Portal Vein (MPV) divides into a larger Right Portal Vein (RPV) and a small Left Portal Vein (LPV). The RPV then divides into Right Anterior Portal Vein (RAPV), supplying to segments V and VIII and Right Posterior Portal Vein (RPPV) supplying to segments VI and VII. The LPV runs horizontally to left, then turns medially, supplying segments II, III and IV and a branch to segment I - caudate lobe (Type 1) [Table/Fig-1a] [10]. This standard branching pattern was observed in approximately 65-80% of the population [6,9].
(a) Drawing showing the standard portal vein anatomy (Type 1); (b) Drawing showing the Portal trifurcation (Type 2); (c) Right posterior portal vein as the first branch of main portal vein (Type 3); (d) Segment VII branch as the separate branch of the right portal vein (Type 4); (e) Segment VI branch as the separate branch of the right portal vein (Type 5);
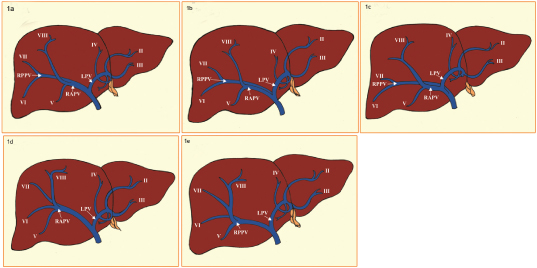
Variations in the portal vein were observed in 20-35% of the population [9,11,12]. The Type 2 variant is the portal trifurcation in which the MPV is divided into right anterior and posterior portal (sectoral) veins and the left portal branch, all arising from a common place, and happens to be commonest variant and was observed in 10.9-15.0% of the population [Table/Fig-1b]. The Type 3 or “Z” anomaly, in which the right posterior portal (sectoral) vein arises directly from main portal vein as its first branch, at the lower part of the hepatic hilum, and the left portal vein is the terminal branch, arising after the origin of the right anterior portal vein. This is the second commonest variant and was seen in 0.3-7.0% of the persons [Table/Fig-1c]. The combined incidence of Type 2 and Type 3 branching patterns has accounted for 8 to 23% of the population [12,13]. The use of 3D reconstruction obtained from thin axial CT images seems to be the most efficient technique in identifying the Type 2 and type 3 anomalies with reported incidences of 35 and 27% respectively [12–14].
The variations in the branching patterns of right portal vein have been reported with incidences ranging from 17-35% [14,15]. The trifurcation of the right portal vein, in which the segment VII branch is the first branch of the RPV and this anomaly was observed in 0.6-2.69% of population (Type 4) [Table/Fig-1d]. The other type of trifurcation in which the segment VI branch arises early as a separate branch from RPV and was seen in 1.34-2.4% of persons (Type 5) [Table/Fig-1e]. In the quadrifurcation of the portal vein, in which two right posterior segmental branches to segments VI and VII, right anterior portal (sectoral) portal vein (RAPV) and the left portal vein, all are offshoots of trunk of the portal vein and was observed in 0.3% of the individuals.
Other rare form of variations include the absence of left portal branch with absence of left lobe (0.3%), absence of right portal branch, with absence of left lobe (0.2-0.3%) and the left portal branch derived from right anterior portal (sectoral) vein without horizontal segment (0.2-0.4%). In extreme cases, a portal branch may vascularize a contralateral segment, the right network feeding segment IV or the left network feeding segment VIII and there was no anastomosis between these networks. However, these variations are less frequent [14,15]. Variations in the left portal vein are very rare and may involve the segmental branches. In addition, congenital malformations such as agenesis of portal vein or its segmental branches, a preduodenal portal vein, duplication of the portal vein, the portal vein communicating with the vena cava or aneurysm of the portal vein were also reported in few cases [16].
Covey AM et al., and Cheng YF et al., while analysing the PV variants using CT scan, accounted only 35% incidence in PV variations [12,17]. Koc et al., retrospectively evaluated 1384 patients using MDCT, had reported 27.4% incidence of PV variants [13], while Schmidt et al., had accounted 20-35% incidence and Atasoy et al., had reported a higher incidence of 34.5% of PV variants [6,14]. The reported incidences of PV variants shows wide discrepancies, which may be due to the use of different sample sizes and variations in the radiological techniques used to outline the portal anatomy. Infact CT-scan, MRI or MRA are very reliable non invasive techniques with same precision in vascular mapping [12].
Hepatic Segmental Anatomy
The liver is composed of eight vascular segments, which have their own arterial and portal venous supply, hepatic venous and biliary drainage. In major liver surgeries, transection of liver parenchyma was effected along the boundaries of these segments, so understanding of segmental anatomy and terminology used is essential in various interventional procedures. Several systems of classification of liver segmentation have been proposed without any standardized terminology to describe liver segments. Goldsmith and Woodburne have divided the liver into four segments based on the second order portal vein branching [18]. Couinaud divided the liver into eight segments based on third order portal vein branching, and this classification system is widely used worldwide [1].
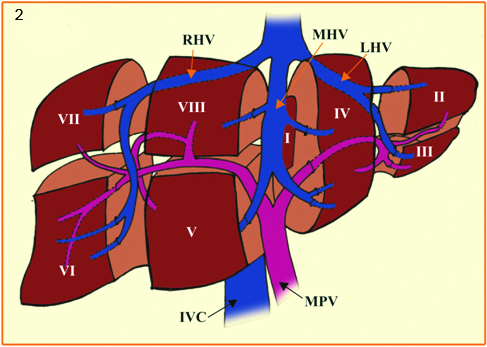
The terminology committee of the International Hepato-Pancreato Biliary Association (IHPBA), in 2000 proposed a standardized terminology for liver segmentation and hepatic resections [19]. The IHPBA described the segmental liver anatomy according to the first, second, and third order branching patterns of the bile ducts and hepatic arteries. The first order division divides liver into the left and right hemilivers. This watershed border is referred as mid-plane of the liver and passes above through the plane of the middle hepatic vein and below through inferior vena cava and fossa for gall bladder, also known as Cantlie’s line. The second-order division demarcates the section/sector, divides the liver into four sections. The right liver is divided into right anterior (segment V and VIII) and right posterior sections (segment VI and VII). The left liver is divided into left lateral (segment II and III) and left medial sections (segment IV). The segment 1, also called as Spigel’s lobe defined between fissure for ligamentum venosum and Cantlie’s line. It is little different in that, it consists of part of the right and left livers due to multiple vascular pedicles, its venous anastomosis and its direct drainage into the inferior vena cava, accounting for its hypertrophy in Budd-Chiari syndrome [20]. In practice, the terms “quadrate lobe” and “caudate” are often used incorrectly; the lower and anterior part of segment IV, also named as IVB is the quadrate lobe and left lateral portion of the segment I is designated as caudate lobe. The third order division of portal vein denotes the individual segments of the liver and are referred to as segments 1-8 and are separated by intersegmental planes [Table/Fig-2].
Drawing showing the Couinaud’s liver segmentation
RHV - Right hepatic vein; LHV - Left hepatic vein; MHV - Middle hepatic vein; RPV - Right portal vein; LPV - Left portal vein; MPV - Main portal vein; RAPV - Right anterior portal vein; RPPV - Right posterior portal vein; IVC - Inferior venae cava
The hepatobiliary surgeries follow directly the anatomic terminology used in IHPBA proposal. A resection involving first order division is called right or left hepatectomy. The resection of segments V-VIII was included in the right hepatectomy (hemihepatectomy) whereas resection of segments II-IV was done in the left hepatectomy. Based on the number of resections such as those involving a single section is called sectionectomy and the extended resections of three sections are called trisectionectomies. Segmentectomy is the resection involving a single segment while the resection of any two contiguous segments is called bisegmentectomy. The right trisegmentectomy, also known as right lobectomy or extended right hepatectomy involves resection of all segments lateral to the umbilical fissure (segments IV-VIII), whereas left trisegmentectomy also known as extended left hepatectomy includes resection of all liver medial to the umbilical fissure and a portion of the right liver(segments II-IV and segments V and VIII) [21,22]. So the usage of Brisbane 2000 terminology of liver anatomy and resections has been encouraged to avoid confusion in referring liver segments or hepatic resections.
Surgical Implications
The pre surgical awareness of the PV variations is clinically important in identifying the accurate location of liver lesions, in the selection of donors in liver transplantations, in tumour resections, in PVE and in TIPS, as portal vein, along with hepatic veins, determines the segmental anatomy. With the increase in percutaneous hepatobiliary interventions and complex surgical resections, a thorough understanding of the PV variants anatomy through pre-procedural cross-sectional imaging will drastically reduce unwanted surgical complications [5,12].
Portal Vein Embolization
The PVE is a modern vascular intervention procedure and is carried out before major hepatectomy, in order to increase the size of the liver that was left in place by the surgeon, before the surgery. This is done approximately four weeks before surgery by embolizing the liver branches that has to be ultimately resected [6]. Hepatobiliary surgeons normally prefer at least 25% (in case of background healthy liver) and 40% (in diseased liver) as Future Liver Remnant (FLR) after hepatectomy. When the expected FLR is low, PVE is performed in an attempt to increase FLR volume [6]. Therefore for a successful major hepatectomy, an adequate FLR is always essential. Thus, the PVE is the gold standard, safe and efficient technique for producing adequate liver hypertrophy in maintaining the FLR, in a planned liver resection [23]. But the major disadvantage is that the liver neoplasms grows continuously without inhibition during the interval period, and eventually make the patient unsuitable for resection in the case of tumours that are in close proximity to major biliary and vascular structures [23].
In order to document the extent of disease, the FLR size and the portal venous anatomy, a cross-sectional imaging for procedural planning is performed prior to PVE. The PVE can be performed using an ipsilateral or contralateral approach and different embolic materials may be used. The anatomic variations increase the complexity of the procedure. In the case of type 3 portal bifurcation, a reversed curved catheter may be required for segments V and VI in the contralateral approach. The ipsilateral approach is usually preferred to safeguard the non diseased portion of liver. So the interventional radiologist must have a precise knowledge of intrahepatic segmental anatomy to perform PVE safely and efficaciously when an atypical PVE is planned [24].
Liver Resection
Major surgical procedures such as right/left trisegmentectomy (extended right/left hepatectomy) require embolization of both right and left PV branches. In the case of an extended right hepatectomy, the embolization of segment IV branch results in better regeneration of segments I, II, III. So a detailed evaluation of PV branching pattern is required preoperatively during these complicated procedures to avoid reflux of the embolizing material into branches of future remnant liver tissue in case, of Type 3 PV variation, if the surgeon ligates only the right anterior branch, there is risk of active bleeding from the patent right posterior branch. So for a safe and clean hepatectomy, a complete obliteration of the portal branches supplying those particular segments is required. Identifying the right portal vein variants in patients who have to undergo left trisegmentectomy can alert the surgeon and avoid a potentially life threatening situation [25].
Liver Transplantation
A precise preoperative analysis of PV variants is mandatory, to perform a split liver/living donor transplantation. The Type 2 (trifurcation) and Type 3 PV variants are much relevant in liver transplantation surgery. The Type 2 trifurcation anomaly shows a complex and difficult intraoperative clamping. The surgical importance of Type 3 variant affects both donor and recipient uniformly. When two portal vein anastomoses are to be performed on two different veins in the recipient, the complete vascularization of the remnant liver in the donor are the events of importance, which increase the complexity of the surgical procedure. In the case of segmental variations, the resection of right or left liver together with its PV branch may devascularize a particular segment (segment IV and VIII) [5,26].
Transjugular Intrahepatic Portosystemic Shunt
The TIPS is the placement of a stent connecting portal vein with hepatic vein, and the procedure depends on the blind canalization of the portal vein by a puncture originating from hepatic vein. A successful TIPS should be created between right hepatic vein and right portal vein. In standard Type 1 PV anatomy, the PV lies at a predictable position relative to the hepatic vein, accounting for high success rates. So an accurate knowledge of anatomical variations of PV is essential for the successful creation of TIPS. The clinical implication of Type 2 and Type 3 variants produces an altered spatial relationship between these vessels, so one main larger right trunk may not be available and thus resultant target may be smaller in caliber [27]. So we advocate cross-sectional imaging before and during the TIPS procedure to assess the venous anatomy to have a good success rate and to avoid complications.
Segmental Localization of Hepatic Lesions
The exact localization of lesions, using Couinaud’s segmentations is of utmost important to facilitate the identification, for a follow up, a biopsy, an interventional procedure or a surgical removal. The difficulty arises when a lesion is placed at the edges of different segments, especially in the hepatic dome, where the precision is very poor. In some patients, the lesions located postero-superior to the right hepatic vein may belong to segment VIII, rather than to segment VII and similarly segment IV lesion may extend into segment VIII. In case of difficulties in CT scans, the use of thick MIP slices, 3D reconstructions or MR imaging with MPR and 3D reconstructions of vessels may provide a better segment localization of focal hepatic lesions and predict the type of resection [28]. Hence the portal vein along with hepatic veins determines the segmental anatomy, and the awareness of PV variations is important in identifying the location of liver lesions.
Associated Liver Partition and Portal vein ligation for Staged Hepatectomy
The surgical resection with negative margins is the only potentially curative treatment in majority of patients with both primary and secondary carcinomatous liver disease. Due to the unique regenerating efficiency of liver cells, 20% of the non tumoural liver remnant volume was spared to avoid the post surgical liver failure [29]. If the person has chemotherapy induced liver injury, the FLR should be at least 30% of the total volume; but in the presence of cirrhosis, a 40% FLR is advisable [29]. Recently newer innovative approaches have been developed that are the variations of the standard PVE, to increase the resectability of the tumours that are too advanced to be resected leaving a sufficient FLR. The right PVE and preoperative or intraoperative ligation of the right portal vein (PVL) are the two techniques most commonly used to induce FLR hypertrophy in patients with inadequate FLR. The PVL is based on the occlusion of the flow in one of the main branches of the portal vein inducing atrophy in the ipsilateral liver and subsequent hypertrophy of the contralateral lobe. Due to the larger volume of the right liver, usually the right branch of the portal vein is occluded to increase the volume of the left liver. These two above methods produce an equivalent amount of liver hypertrophy in the range of 10-46% within 2-8 weeks time intervals [30,31]. Due to the longer period of time required for the compensatory hypertrophy and with its decreased amount of liver hypertrophy, the above two techniques drastically reduce the operability in patients with fast growing liver tumours. Thus the ALPPS, a new and advanced form of hepatic resection, is performed in two stages. The first stage of the procedure is the intraoperative ligation of the right portal branch to the partition of the liver, following the procedure of an extended right hepatectomy. The diseased part of the liver is left in situ and remains vascularized by the right hepatic artery, while the biliary and systemic venous drainage through the right biliary duct and hepatic veins are preserved [32]. The second step of the procedure is performed 1-2 weeks after the first; the diseased part of the liver is removed by sectioning the remaining biliary, hepatic arterial and systemic venous pedicles. This step-wise ALPPS permits a faster hypertrophy of the FLR and also ensure wider operability than previous techniques. Even though the ALPPS has the advantage of higher hypertrophy rates than the standard PVE, the mortality and morbidity rates were significantly higher, which limit its applications currently [33,34].
Radioembolization/SIRT
The SIRT or the radioembolization with Yttrium-90, a recently advanced form of treatment modality is widely used for treating locally advanced liver tumours. The reported radiological tumour response rate of the SIRT procedure is between 42-70% [35]. The selective application of the unilobar SIRT not only results in significant contralateral liver hypertrophy, but also has an efficient local tumour control. It is relatively safe than PVE, increases the resectability rates in patients with malignant liver diseases and also increases the rate of FLR hypertrophy and thus permits tumour down staging [36,37]. The Y-90 SIRT also has the advantage of providing both tumour control and increasing the FLR size in patients with a large tumour mass that surrounds the major biliary/vascular structures and where the ability to achieve adequate oncological free margins are needed [36]. The mechanism of hypertrophy is the changes in the liver parenchyma, consistent with portal hypertension following Y-90 SIRT. These include increase in portal vein and spleen diameter with corresponding decrease in platelet count. Thus, the post Y-90 SIRT hypertrophy provides a novel and exciting option in the multidisciplinary management of patients with liver tumours and is often utilized in a palliative setting, on patients with inferior functional reserve and liver function [38].
Conclusion
The preoperative PVE is indicated for major hepatic resection. It has become the standard care for patients with hepatic malignancies. The portal vein variants are commonly observed in routine CT examinations and in triphasic MDCT with axial-oblique and coronal oblique thin slice images with MPR, MIP reformations. The hepatobiliary surgeons and interventional radiologists must be familiar with the segmental anatomy of the liver, have a good knowledge of portal vein anatomy and its branching variations and also shall understand the different techniques employed to ligate the portal vein during planned hepatic resection. The PVE is performed using different surgical techniques with different embolic materials to increase the degree of liver hypertrophy and consequently the size of FLR. As it is a supplementary procedure to a major hepatic resection, importance must always be given to safety without compromising the integrity of the FLR and a close coordination between surgeons and radiologists is always essential for successful surgical outcomes. A knowledge regarding the advantages and disadvantages of these approaches and of the various embolic materials used is essential to best tailor the procedure and to reduce the procedure related morbidity and mortality. Despite these alternatives, the PVE still remains as the gold standard method, as it is minimally invasive and avoids a laparotomy to allow for hypertrophy of the contralateral liver and make potentially unresectable lesions resectable by better selection and lower risk of postoperative liver insufficiency or failure.
[1]. Couinaud C, Le foie. Etudes anatomiques et chirurgicalesThe Liver. Anatomical and surgical investigations 1957 ParisMasson [Google Scholar]
[2]. Ryu M, Cho A, New Liver Anatomy 2009 Tokyo, Berlin, Heidelberg, New YorkSpringer [Google Scholar]
[3]. Bismuth H, Revisiting liver anatomy and terminology of hepatectomiesAnn Surg 2013 257:383-86. [Google Scholar]
[4]. Couinaud C, Erreurdans le diagnostic topographique des lesions hepatiquesAnn Chir 2002 127:418-30. [Google Scholar]
[5]. Erbay N, Raptopoulos V, Pomfret EA, Kamel IR, Kruskal JB, Living donor liver transplantation in adults: Vascular variants important in surgical planning for donors and recipientsAJR 2003 181:109-14. [Google Scholar]
[6]. Schmidt S, Demartines N, Soler L, Schnyder P, Denys A, Portal vein normal anatomy and variants: Implication for liver surgery and portal vein embolizationSemin Intervent Radiol 2008 25:86-91. [Google Scholar]
[7]. Langman J, Sadler T, Medical Embryology 2011 11th editionLippincott Williams and Wilkins, South Asian Edition, Wolters Kluwer India:192-193. [Google Scholar]
[8]. Collardeau-Frachon S, Scoazec JY, Vascular development and differentiation during human liver organogenesisAnat Rec 2008 291:614-27. [Google Scholar]
[9]. Cheng Y, Huang T, Lee T, Chen T, Chen C, Variation of intrahepatic portal vein; angiographic demonstration and application in living related hepatic transplantationTransplant Proc 1996 28:1667-68. [Google Scholar]
[10]. Blumgart LH, Surgery of the liver and biliary tract 2000 Philadelphia, PASaunders:1640-1645. [Google Scholar]
[11]. Couinaud C, Liver anatomy: portal (and suprahepatic) or biliary segmentationDig Surg 1999 16:459-67. [Google Scholar]
[12]. Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT, Incidence, patterns, and clinical relevance of variant portal vein anatomyAJR Am J Roentgenol 2004 183:1055-64. [Google Scholar]
[13]. Koc Z, Oguzkurt L, Ulusan S, Portal vein variations: Clinical implications and frequencies in routine abdominal multidetector CTDiagn Interv Radiol 2007 13:75-80. [Google Scholar]
[14]. Atasoy C, Ozyurek E, Prevalence and types of main and right portal vein branching variations on MDCTAJR Am J Roentgenol 2006 187:676-81. [Google Scholar]
[15]. Wu TC, Lee RC, Chau GY, Chiang JH, Chang CY, Reappraisal of right portal segmental ramification based on 3-dimensional volume rendering of computed tomography during arterial portographyJ Comput Assst Tomogr 2007 31:475-80. [Google Scholar]
[16]. Germain T, Favelier S, Cercueil JP, Denys A, Krause D, Guiu B, Liver segmentation: Practical tipsDiagn interv imag 2004 95:1003-16. [Google Scholar]
[17]. Cheng YF, Huang TL, Chen CL, Sheen-Chen SM, Lui CC, Chen TY, Anatomic dissociation between the intrahepatic bile duct and portal vein: Risk factors for left hepatectomyWorld J Surg 1997 21:297-300. [Google Scholar]
[18]. Goldsmith NA, Woodburne RT, The surgical anatomy pertaining to liver resectionSurg Gynecol Obstet 1957 105:310-18. [Google Scholar]
[19]. Strasberg SM, Belghiti J, Clavien P, Gadzijev E, Garden JO, Lau W, The Brisbane 2000 terminology of liver anatomy and resectionsHPB 2000 2:333-39. [Google Scholar]
[20]. Ortale J, Borgers Keiralla L, Anatomy of the portal branches and hepatic veins in the caudate lobe of the liverSurg Radiol Anat 2004 26:384-91. [Google Scholar]
[21]. Blumgart LH, Belghiti J, Surgery of the liver, biliary tract, and pancreas 2007 4th edPhiladelphia, PASaunders Elsevier [Google Scholar]
[22]. Strasberg SM, Nomenclature of hepatic anatomy and resections: A review of the Brisbane 2000 systemJ Hepatobiliary Pancreat Surg 2005 12:351-55. [Google Scholar]
[23]. Pamecha V, Levene A, Grilio F, Woodward N, Dhilion A, Davidson BR, Effect of portal vein embolization on the growth rate of colorectal liver metastasesBr J Cancer 2009 100:617-22. [Google Scholar]
[24]. Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA Jr, Ahrar K, Transhepatic portal vein embolization: Anatomy, indications, and technical considerationsRadiographics 2002 22:1063-76. [Google Scholar]
[25]. D’Angelica M, Fong Y, The liver. 18th ed. In: Courtney J, Townsend M, Beauchamp RD, Evers BM, Mattox KL., editorsSabiston Textbook of Surgery 2008 PhiladelphiaSaunders:1512-15. [Google Scholar]
[26]. Sureka B, Patidar Y, Bansal K, Rajesh S, Agrawal N, Arora A, Portal vein variations in 1000 patients: Surgical and radiological importanceBr J Radiol 2015 88(1055):20150326 [Google Scholar]
[27]. Saad N, Darcy M, Saad W, Portal anatomic variants relevant to transjugular intrahepatic portosystemic shuntTech Vasc Interv Radiol 2008 11:203-07. [Google Scholar]
[28]. Soyer P, Bluemke D, Bliss D, Woodhouse C, Fishman E, Surgical segmental anatomy of the liver: Demonstration with spiral CT during arterial portography and multiplanar reconstructionAJR Am J Roentgenol 1994 163:99-103. [Google Scholar]
[29]. Adams RB, Alola TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN, Selection of hepatic resection of colorectal liver metastases: Expert consensus statementHPB 2013 15:91-103. [Google Scholar]
[30]. Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Portal vein embolization before right hepatectomy: Prospective clinical trialAnnals of Surgery 2003 237:208-17. [Google Scholar]
[31]. Vivarelli M, Vincenzi P, Montalti R, Fava G, Tavio M, Coletta M, ALPPS procedure for extended liver resections: A single centre experience and a systematic reviewPLoS ONE 2015 10(12):e0144019 [Google Scholar]
[32]. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settingsAnnals of Surgery 2012 255:405-14. [Google Scholar]
[33]. Aloia TA, Associating liver partition and portal vein ligation for staged hepatectomy: Portal vein embolization should remain the gold standardJAMA Surg 2015 150:927-28. [Google Scholar]
[34]. deSantibanes E, Ardiles V, Alvarez FA, Associating liver partition and portal vein ligation for staged hepatectomy: A better approach to treat patients with extensive liver diseaseJAMA Surg 2015 150:929-30. [Google Scholar]
[35]. Khor AY, Toh Y, Allen JC, Ng DC, Kao YH, Zhu G, Survival and pattern of tumour progression with yttrium-90 microsphere radioembolization in predominantly hepatitis B Asian patients with hepatocellular carcinomaHepatol Int 2014 8:395-404. [Google Scholar]
[36]. Teo JY, Goh BK, Cheah FK, Allen JC, Lo RH, Ng DC, Underlying liver disease influences volumetric changes in the spared hemiliver after selective internal radiation therapy with (90)Y in patients with hepatocellular carcinomaJ Dig Dis 2014 15:444-50. [Google Scholar]
[37]. Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long term outcomesGatroenterology 2010 138:52-64. [Google Scholar]
[38]. Teo JY, Allen JC, Ng DC, Choo SP, Tai DWM, Chang JPE, A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90HPB 2016 18:7-12. [Google Scholar]