Diabetes Mellitus (DM) has assumed the proportions of a global epidemic and the number of diabetics in the world today is 415 million. This number is being projected to increase to 642 million by 2040 [1]. India leads the world in terms of its diabetic population and estimates have revealed that there are 62.4 million people with diabetes, and 77.2 million with prediabetes in India [2]. It is predicted that by 2030, DM may affect up to 79.4 million in India [3]. Due to these sheer numbers, the socioeconomic burden as a result of diabetes in India is among the highest in the world [4].
Along with this rapidly increasing disease, there is an increase in the associated microvascular complication DR, which is said to affect more than 93 million people worldwide [5]. Almost all patients with Type 1 DM and over 60% of the patients with Type 2 DM will develop DR in 20 years. DR is the most frequent cause of preventable blindness in middle aged population and therefore, represents a major public health issue [6].
Oxidative stress is considered to be one of the key factors in the pathogenesis of DR [7]. The retina is especially more susceptible to oxidative damage because of its high energy demands and exposure to light [8]. Various mechanisms contributing to the pathogenesis of DR such as inflammation, polyol pathway, accumulation of Advanced Glycation End Products (AGEs), flux of hexosamine Pathway and Protein Kinase C (PKC) activation are associated with overproduction of Reactive Oxygen Species (ROS) by the mitochondria [9].
Although, bilirubin has long been considered as a toxic waste product of heme catabolism, its role as a potential endogenous anti-oxidant under physiological conditions is being increasingly recognized [10]. Apart from its ability to scavenge over produced ROS, bilirubin is also said to have anti-inflammatory effects [11]. Based on these physiological effects of bilirubin, several population-based studies have reported a negative association between serum bilirubin concentrations and the risk of diabetes [12], coronary artery disease [13], chronic kidney disease [14], peripheral arterial disease [15] and diabetic peripheral neuropathy [16].
Few studies in literature have shown, the association between serum bilirubin and DR [17–20]. Apart from age, gender, smoking and intake of drugs serum bilirubin concentrations are also affected by factors such as altitude and ethnicity and these are likely to influence the biological effects of bilirubin on human body.
Considering the potential role of oxidative stress in the pathogenesis of DR and the antioxidant and cytoprotective effects of bilirubin, this cross-sectional study aimed to evaluate the association between serum bilirubin and severity of retinopathy in patients with Type 2 DM from Southern India.
Materials and Methods
This was a hospital-based cross-sectional study which included patients with Type 2 DM who were referred to the Ophthalmology Department of a tertiary care centre in Southern India between January 2014 and December 2014 for screening of DR. Exclusion criteria included history of chronic alcohol intake, smoking, intake of hepatotoxic medications within past six months, preexisting hepatobiliary abnormalities or chronic liver disease. Patients with preexisting ocular diseases like glaucoma, high myopia, previous ocular surgery or photocoagulation were excluded from the study. Patients with hypertension (>160/90 mmHg), anaemia or other systemic diseases like nephropathy which can accelerate the progression of DR were also excluded from the study. The study protocol was approved by the Institutional Ethics Committee and this study was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants.
Information on age, gender, duration of diabetes, blood pressure and body mass index was obtained from all participants.
All participants underwent a complete ocular examination including fundus examination with slit lamp biomicroscope using a 90 diopter lens after dilatation of pupils using 1% tropicamide eyedrops. This was followed by 30 degree colour stereoscopic fundus photography of 7 standard Early Treatment Diabetic Retinopathy Study (ETDRS) fields using a digital fundus camera (TRC-50 DX Mydriatic retinal camera; Topcon Medical Systems, Oakland, NJ). The severity of DR was determined by grading of colour fundus photographs following the international clinical disease severity scale for DR which is based upon the findings of WESDR (Wisconsin Epidemiological Study of Diabetic Retinopathy) and ETDRS [21].
The diabetic patients were assigned to one of the following groups based on presence and severity of retinopathy.
Diabetic patients with no retinopathy.
Mild Non-Proliferative Diabetic Retinopathy (NPDR).
Moderate NPDR.
Severe NPDR and
Proliferative Diabetic Retinopathy (PDR).
The eye with worse retinopathy was chosen for inclusion in the study examinations. If both eyes had equal retinopathy, the right eye was assigned to the study.
Fasting venous blood samples were collected for the determination of serum total, direct and indirect bilirubin, blood glucose and Glycosylated Haemoglobin (HbA1c). The normal range of total serum bilirubin levels used in the study was 0.2 to 1.2 mg/dl.
Statistical Analysis
Statistical analysis was performed using SPSS version 19. The baseline characteristics of patients were presented as mean±standard deviation and n (%). Data on the patient’s clinical characteristics were compared using One-way Analysis of Variance (ANOVA) as well as an independent sample Student’s t-test. The serum bilirubin levels were divided into quartiles and Chi-square test was used to see the comparison between the proportions. Multiple logistic regression analysis was performed to assess independent associations between the presence of key risk factors of DR including the statistically significant factors in univariate analysis. All tests for statistical significance were two-tailed, and performed assuming a Type I error probability of <0.05.
Results
A total of 116 subjects were included in the study out of whom 86 were patients with Type 2 diabetes and 30 were controls.
The mean total bilirubin level among diabetic subjects (0.52±0.17) and controls (0.51±0.19) were found to be similar. The direct bilirubin level was found to be higher in diabetics (0.29±0.13) as compared to controls (0.18±0.06, p=0.000). The indirect bilirubin level was lower in diabetics (0.23±0.07) as compared to healthy controls (0.33±0.13, p=0.000).
[Table/Fig-1] shows the clinical characteristics of the study population. The mean duration of diabetes and HbA1c were significantly higher among patients with DR compared to those without retinopathy (p<0.001). We compared the serum total, direct and indirect bilirubin levels for diabetics without retinopathy (n=24) and those with DR (n=62). The total bilirubin was 0.65±0.16 in patients with no retinopathy and 0.47±0.16 in patients with DR. The mean total as well as direct bilirubin levels were found to be lower in patients with retinopathy as compared to no retinopathy group (p<0.001). There was no statistically significant difference in the levels of indirect bilirubin among both groups (p=0.100).
Showing the clinical characteristics of study population.
| Characteristic | Controls (n=30) | Diabetics with no retinopathy (n=24) | Diabetics with retinopathy (n=62) |
|---|
| Age (yr) | 52.97±8.95 | 57.63±10.54 | 57.29±8.5 |
| Females, no. (%) | 18 (60%) | 9 (37.5%) | 23 (37.1%) |
| Duration of diabetes (yr) | - | 3.67±1.20 | 6.74±3.51* |
| Body mass Index (Kg/m2) | 28.1±0.7 | 27.8±0.4 | 28±0.7 |
| Systolic blood pressure (mm Hg) | 120.1±2.7 | 117.1±1.6 | 122.6±2.9 |
| Diastolic blood pressure (mm Hg) | 79.7±2.1 | 74.1±1.2 | 73.3±2.4 |
| HbA1c (%) | 5.72±0.35 | 8.09±0.78 | 9.75±1.74* |
| Total bilirubin (mg/dl) | 0.51±0.19 | 0.65±0.16 | 0.47±0.16* |
| Direct bilirubin (mg/dl) | 0.18±0.06 | 0.39±0.13 | 0.25±0.10* |
| Indirect bilirubin (mg/dl) | 0.33±0.13 | 0.25±0.05 | 0.22±0.07 |
* p-value < 0.05; HbA1c- Haemoglobin A1c
Of the 62 diabetic patients with retinopathy, 15 patients had mild NPDR, 22 had moderate NPDR and 9 had severe NPDR. PDR was noted in 16 patients. We divided the study participants into quartiles on the basis of serum total bilirubin concentration (quartile 1=<0.40 mg/dl, quartile 2= 0.41-0.50 mg/dl, quartile 3= 0.51-0.60 mg/dl, quartile 4=>0.61 mg/dl).
[Table/Fig-2] shows the correlation between serum total bilirubin and severity of DR.
Showing the correlation between serum total bilirubin and severity of diabetic retinopathy.
| Quartiles of serum total bilirubin (mg/dl) | p-value |
|---|
| <0.40 | 0.41-.50 | 0.51-.60 | >0.61 |
|---|
| Controls | 11 | 10 | 3 | 6 | 0.010 |
| NO DR | 2 | 6 | 5 | 11 |
| Mild NPDR | 5 | 4 | 3 | 3 |
| Moderate NPDR | 8 | 8 | 3 | 3 |
| Severe NPDR | 7 | 1 | 0 | 1 |
| PDR | 11 | 4 | 1 | 0 |
NPDR-Non proliferative diabetic retinopathy; PDR-Proliferative diabetic retinopathy
The percentage of DR in persons with lowest bilirubin quartile was 36.04%, compared with the percentage of 19.8%, 8.1% and 8.1% respectively in the second, third and fourth quartiles. Thus, the proportion of patients with DR was significantly higher in quartile 1 with lower levels of bilirubin as compared to the other three quartiles [Table/Fig-2]. The severity of DR was inversely proportional to the serum bilirubin levels (p=0.010) and none of the patients in the last quartile (>0.61) had proliferative retinopathy.
On multiple logistic regression analysis the duration of diabetes, HbA1c and total serum bilirubin were found to be independently associated with DR [Table/Fig-3]
Showing the odds ratio for association between key risk factors and occurrence of diabetic retinopathy using logistic regression model.
| Risk factor | OR | 95% CI | p-value |
|---|
| Age (years) | 1.03 | 0.960-1.111 | 0.386 |
| Duration of diabetes (years) | 2.05 | 1.172-3.592 | 0.012* |
| HbA1c (%) | 2.42 | 1.177-4.984 | 0.016* |
| Serum total bilirubin (mg/dl) | 0.001 | 0.000-0.054 | 0.001* |
* p-value < 0.05; OR-Odds ratio; CI-Confidence Interval; HbA1c- Haemoglobin A1c
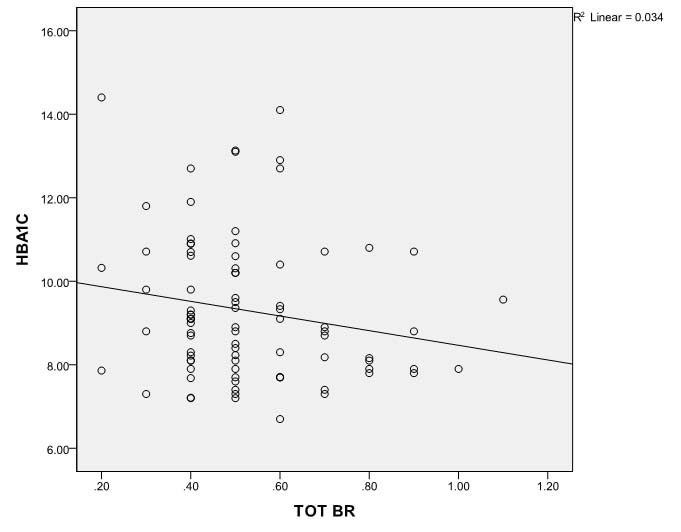
Also, serum total bilirubin in diabetic subjects was found to have negative association with HbA1c [Table/Fig-4].
Scatter diagram showing negative association between serum total bilirubin and HbA1c (glycosylated haemoglobin) in diabetics.
TOT BR-Total bilirubin; HBA1C-glycosylated haemoglobin
Discussion
In the present, study serum bilirubin concentration correlated negatively with the severity of retinopathy in patients with Type 2 DM. Multiple regression analysis also revealed serum total bilirubin as an independent risk factor associated with DR.
Bilirubin is generated when the enzyme Heme Oxygenase (HO) catalyzes the degradation of heme. This leads to the formation of biliverdin which is converted to bilirubin by biliverdin reductase. In the hepatocytes, unconjugated (lipid-soluble) bilirubin is conjugated by uridine diphosphate glucuronyl transferase (UDP-GT) to a water soluble form for excretion. Total bilirubin is the sum of unconjugated (indirect) and conjugated (direct) bilirubin and generally ranges from 0.2 to 1.2 mg/dl in healthy individuals.
Ever since the description of beneficial role of bilirubin as a physiologic antioxidant, several clinical studies evaluating, the possible associations between serum bilirubin and the risk of diabetes (and its complications) have been published [22–24].
In this observational study, we noted that Type 2 diabetics with retinopathy had significantly lower serum bilirubin concentrations than those without retinopathy. Our observations are in line with epidemiologic and hospital based studies elsewhere which have suggested an inverse relation between serum total bilirubin and DR [17–20]. There has been only one study on the association between serum bilirubin and DR from Indian population by Dave et al., [25]. They noted higher levels of total, direct and indirect bilirubin in patients without DR as compared to patients with retinopathy. However, in our study only total and direct bilirubin concentrations were found to be higher in patients without DR. Serum indirect bilirubin concentration was similar in both groups. Most studies on serum bilirubin and DR have focused on only total bilirubin without separating bilirubin types. Limited studies have shown a differentially protective effect of direct bilirubin on metabolic syndrome [26] and chronic kidney disease [27]. Further studies on the relation between bilirubin subtypes and DR are clearly needed.
In this study, we divided bilirubin levels into quartiles (<0.40, 0.41-0.50, 0.51-0.60 and >0.61 mg/dl) and considered the lowest quartile as reference. This approach was to maintain adequate numbers within each quartile. The absolute values of serum bilirubin were low in our study compared to studies by Yasuda et al., and Najam et al., where the value of serum bilirubin in the lowest and highest quartile observed were<0.60 and >0.90 mg/dl respectively [17,18]. There are several potential reasons for the observed findings. Apart from differences in ethnicity, our hospital based study population was very much different from the above said epidemiologic studies in age distribution, anthropometry, comorbidities such as hypertension, cardiovascular disease and smoking habits, all factors that may affect the bilirubin levels and potentially the association between bilirubin and DR. Our study participants were non smokers and had no history of major systemic diseases.
Several potential mechanisms have been proposed for the observed relationship between serum bilirubin concentration and DR. Excessive production of free radicals and resultant oxidative stress has been said to contribute significantly to worsening of DR [28,29]. Bilirubin has been postulated as a potent antioxidant and cytoprotective agent that can protect cells from a 10,000 fold excess of hydrogen peroxide [30]. Therefore, lower serum bilirubin concentration would be expected to be associated with increased oxidative stress and thereby, accelerated progression of DR. Additionally clinical studies have demonstrated elevated levels of inflammatory markers like C-reactive protein and vascular cell adhesion molecules in patients with DR suggesting that inflammation is a critical factor leading to endothelial dysfunction in DR [31,32]. Bilirubin has been proven to have anti-inflammatory properties and in-vitro studies have shown that bilirubin inhibited tumour necrosis factor α –induced upregulation of vascular cell adhesion molecules [33]. Hence, it could be hypothesized that higher serum bilirubin concentration is associated with a lower risk of development and progression of DR through its anti-inflammatory effects. Further, recent animal studies have shown that, increasing serum bilirubin levels either through endogenous synthesis or exogenous administration improves endothelial dysfunction in diabetes by activation of protein kinase B [34].
Considering these physiologic effects of bilirubin it can be postulated that bilirubin can effectively retard or interrupt the pathways to development of DR and therefore serve as a potential biomarker for risk of DR.
We observed an inverse and graded association between serum total bilirubin and severity of DR in this study and the strength of serum total bilirubin concentration as an independent determinant of DR was similar to those of conventional risk factors such as HbA1c and duration of diabetes in multiple regression analysis. Serum total bilirubin concentration was an independent determinant of DR even after adjustment for other risk factors (p=0.001).
We also observed a negative association between serum total bilirubin and HbA1c among diabetic subjects. Our data are consistent with observations of Choi et al., who have also noted a significant inverse association between serum total bilirubin and HbA1c in their study on 690 Korean patients with Type 2 DM [35].
This can be partly explained by the fact that, increased serum bilirubin reduces the oxidative stress and thereby, inhibits the glycosylation of haemoglobin. Also, upregulation of the heme oxygenase system and increased generation of bilirubin/biliverdin have been proven to enhance insulin production and sensitivity in diabetic rats [36]. Therefore, it can be suggested that bilirubin improves glycaemic control through its effects on insulin. Further studies are needed to precisely elucidate the mechanism behind this association.
The strength of our study is based on the fact that our study participants were non-smokers and did not have other systemic diseases. We excluded these patients to focus on the association between serum bilirubin and retinopathy in patients with Type 2 diabetes.
Limitation
Our study has some limitations. First, our sample size was limited and the cross-sectional design of our study prevents determination of a temporal association or the cause-effect relationship between serum total bilirubin and the development of retinopathy. Only prospective studies can reveal whether serum bilirubin predicts the development of DR. Second, we performed only a single measurement of serum bilirubin which might have within subject variation. However, all subjects including controls were examined after overnight fast.
Conclusion
In conclusion, our cross-sectional study suggested that low total serum bilirubin level was independently associated with higher risk of DR in patients with type 2 diabetes, regardless of other conventional risk factors. Further, prospective studies on large samples are required to better assess the effects of bilirubin on DR.
* p-value < 0.05; HbA1c- Haemoglobin A1c
NPDR-Non proliferative diabetic retinopathy; PDR-Proliferative diabetic retinopathy
* p-value < 0.05; OR-Odds ratio; CI-Confidence Interval; HbA1c- Haemoglobin A1c