Chronic Rhino Sinusitis (CRS) is one of the most common health problem which leads to frequent visits to otorhinolaryngologist. Even with the recent advances, the aetiology, pathogenesis and treatment of CRS is a matter of debate [1]. It is a clinical syndrome associated with persistent inflammation of the mucosa of the nose and paranasal sinuses for 12 weeks or longer [2,3]. Nasal Polyposis (NP), a subgroup of CRS has incidence of 4% in general population and 25–30% among CRS patients [1]. Treating them is a challenging task being uncertain aetiology and more chance of recurrence [4]. Diagnosis of CRS depends on the signs, symptoms and objective evidence of mucosal inflammation detected by nasal endoscopy and/or computerized tomography of paranasal sinuses [5]. It is also important to recognize that this is a heterogeneous disease spectrum which is subjected to further subclassifications [6].
Patients with CRS may be divided between CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) which leads to both clinical and pathological differences. Eosinophils, increased histamine, IL-13 and IL-5 mediates CRSwNP [7]. By contrast, CRSsNP seems to be predominantly mediated by neutrophilic inflammation [8].
Exceptionally, some CRSsNP cases may show increased eosinophilic infiltration. So distinction between CRS with and without polyps is unclear. Moreover, sinonasal mucosal inflammation also occurs due to systemic diseases, which should be differentiated well [5]. Cystic fibrosis, sarcoidosis, Wegener’s granulomatosis and Primary Immunodeficiency (PID) may present with sinus involvement as a component of the multisystem process. However, certain tumour, mycetoma and foreign body reaction may also lead to secondary CRS [5].
Previous data showed that blood eosinophilia and the extent of eosinophilic inflammation is related to the extent of sinonasal mucosal involvement, the severity of nasal disease, and size of nasal polyps suggesting that CRS with and without NPs represent two end of a spectrum of chronic inflammatory disease [9,10]. Recently, nasal polyposis is differentiated from CRS by two entities. First, nasal polyposis can be differentiated from CRS on the basis of markers of eosinophilic inflammation such as eotaxin, IL-5, Eosinophil Cationic Protein (ECP) and IgE. Second, CRS can be differentiated from nasal polyposis on the basis of myeloperoxidase, TGF-β1, IFN-γ and TNF-α levels. These patterns are similar to lower airway diseases (COPD/Asthma) which suggest a common aetiological/pathogenetic aspects [11]. Hence, the present study was undertaken to find out the significance of blood eosinophil count levels in CRS with nasal polyp and to compare blood eosinophil count and eosinophil count in the histopathology of the polyps.
Materials and Methods
In this retrospective study, 63 patients who underwent endoscopic sinus surgery for CRS with nasal polyps at Amrita Institute of Medical Sciences, Kochi, Kerala, India from August 2012 to November 2014 were reviewed through medical records. Study was approved by the instituitional ethics committee at Amrita Institute of Medical Sciences, Kochi, in November 2012. The demographic data, clinical examination details regarding preoperative nasal endoscopies, MDCT PNS without contrast, blood eosinophil counts and the histopathology of polyps were collected for each of the cases and were systematically recorded in a proforma. The groups were divided based on the number of patients suffering from non eosinophilic rhino sinusitis (Group 1) and those from eosinophlic rhino sinusitis (Group 2). For estimating the sample size, the most important variable, blood eosinophil percentage was monitored. Since no Indian studies could be located on the variables, minimum sample size was estimated based on the studies done abroad. The sample size was calculated based on a standard formula for comparing two Groups for each variable with a confidence level of 99% and power 99%. Accordingly, minimum sample size of 25 was obtained for each group for the present study. In this study, rhino sinusitis was defined as per European Position paper on Rhino sinusitis and Nasal Polyp 2007 [12]. After a positive history and examination findings, patients were asked to undergo MDCT PNS and the severity of the disease was assessed by CT scoring based on Lund Mackay Scoring System (2011) [13].
Following this, the absolute blood eosinophil count was estimated. The Clinical Diagnostic indicators for eosinophilic CRS in this study were:
1. Bilateral sinonasal polyposis;
2. CT scan, Lund Mackay score, Mean ethmoid sinus score ≥1, Predominant opacification of ethmoid sinus (mean ethmoid sinus score more than mean maxillary sinus score);
3. Peripheral blood eosinophil count more than normal;
Prior to surgery, all cases underwent routine diagnostic nasal endoscopies. Following Endoscopic sinus surgery, the biopsy of the polyp was sent for histological study. The samples were fixed with formaldehyde solution and later with haematoxylin and eosin staining. Slides were examined under 10X and 400X and then the number of eosinophils were counted under 5 random high power fields. Eosinophilic Chronic Rhinosinusitis (ECRS) was histopathologically defined when tissue eosinophil was>10/HPF [14]. Both the counts (eosinophil blood count and histopathological eosinophil count) were then compared to know the significance of eosinophil count in CRS with nasal polyp.
Inclusion/Exclusion criteria
Patients diagnosed as CRS with nasal polys and between 16 to 80 years were included in the study. Patients with proven acute infection, cystic fibrosis, primary ciliarydyskinesia, fungal sinusitis, gastroesophageal reflux diseases were excluded from the study.
Statistical Analysis
For all continuous variables the results was given in mean±standard deviation. To compare the means of continuous variables between the groups, student’s independent sample t-test/Mann Whitney test was performed. Probability value (p-value) less than or equal to 0.05 was considered as statistically significant. All test of statistical significance were 2 tailed.
Results
A total of 63 patients who presented with CRS and nasal polyps between 2012 to 2014 were studied. Of the 63 cases included, 30 were non eosinophilic which were termed as Group 1 and 33 were eosinophilic termed as Group 2.
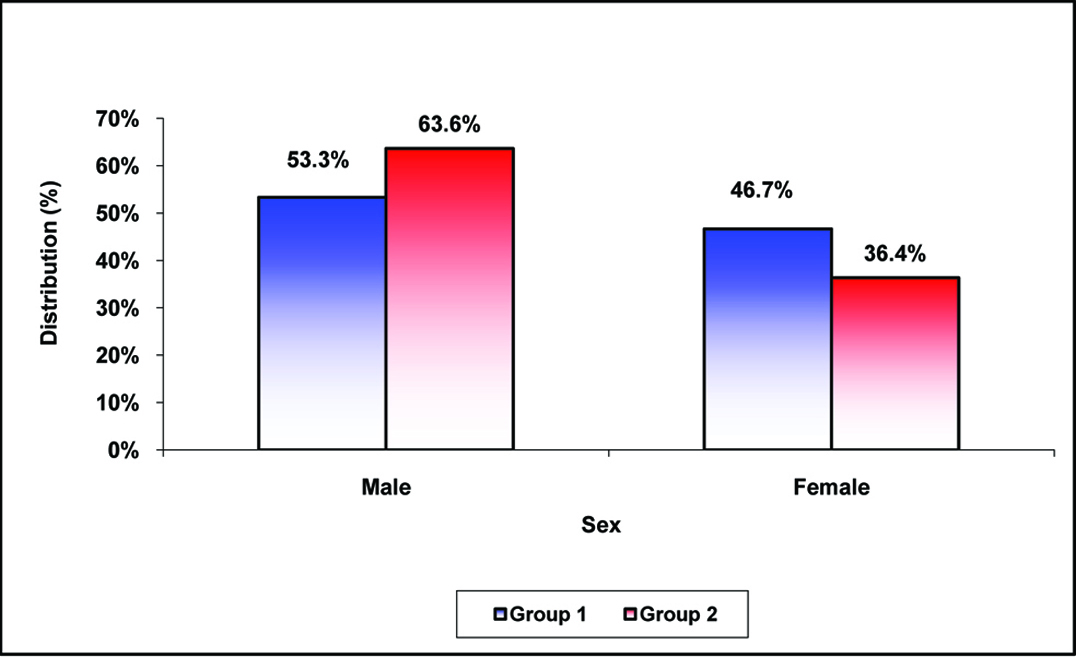
In the present study, among the patients with Group 1, the male to female ratio was found to be 1.14:1 with 53.3% males and in Group 2, the same were noted as 1.75:1 and 63.6% respectively showing a male preponderance for the disease [Table/Fig-1].
In this study, with regard to clinical features, significantly higher number of patients in Group 2 presented with nasal block (97% vs 46.7%; p<0.001), nasal obstruction (90.9% vs 46.7%; p<0.001), nasal discharge (81.8% vs 56.7%; p=0.030), hyposmia (97% vs 30%; p<0.001), asthma (69.7% vs 3.3%; p<0.001). However, clinical features including facial pain (66.7% vs 81.8%; p=0.168) and paranasal sinus tenderness (53.3% vs 54.6%; p=0.923) were comparable in Group 1 and Group 2.
In this study, though 82.9% of the patients had abnormal CT findings in osteomeatal complex among the patients with Eosinophilic Chronic Rhinosinusitis (ECRS), a comparable subset of patients had similar findings in non-ECRS (76.7%) Group and the difference was statistically not significant (p=0.325).
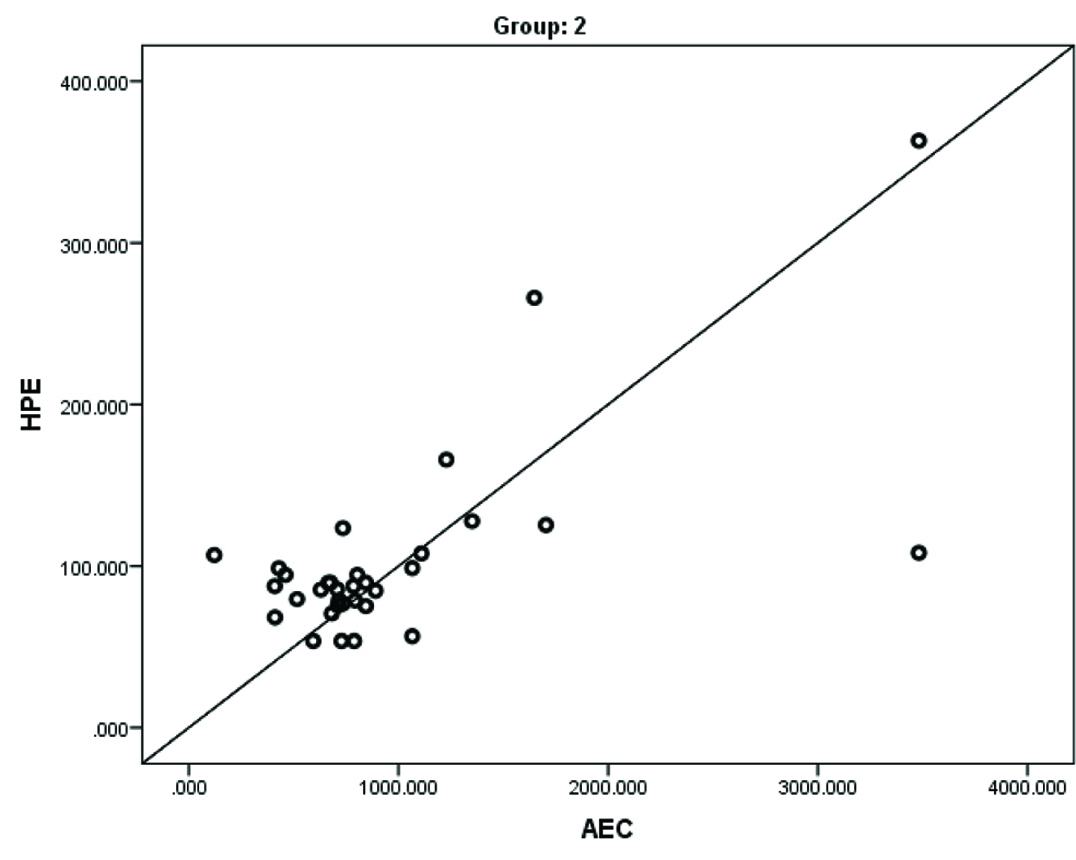
A significantly higher mean EC (3.6±1.90 vs 10.9±3.80; p<0.001), AEC (283.9±167.00 vs 964.2±729.30; p<0.001) and HPE (61.2±23.70 vs 103.10±60.60; p=0.001) levels were noted in Group 2 compared to Group 1 [Table/Fig-2]. There was a strong positive linear correlation between Absolute Eosinophil Count (AEC) and HPE in Group 1 (r=0.686; p<0.001) and Group 2 (r=0.674; p<0.001) [Table/Fig-3,4].
Comparison of mean EC, AEC and HPE.
| Variables | Group 1 Mean±SD | Group 2 Mean±SD | p-value |
|---|
| EC | 3.6±1.90 | 10.9±3.80 | <0.001 |
| AEC | 283.9±167.00 | 964.2±729.30 | <0.001 |
| HPE | 61.2±23.70 | 103.1±60.60 | 0.001 |
Mean Eosinophil count (EC), Absolute Eosinophil count (AEC) and Histopathology Eosinophil Count (HPE) were significantly high in Group 2 compared to Group 1
Correlation of Absolute Eosinophil Count (AEC) and histopathology eosinophil count in Group 2.
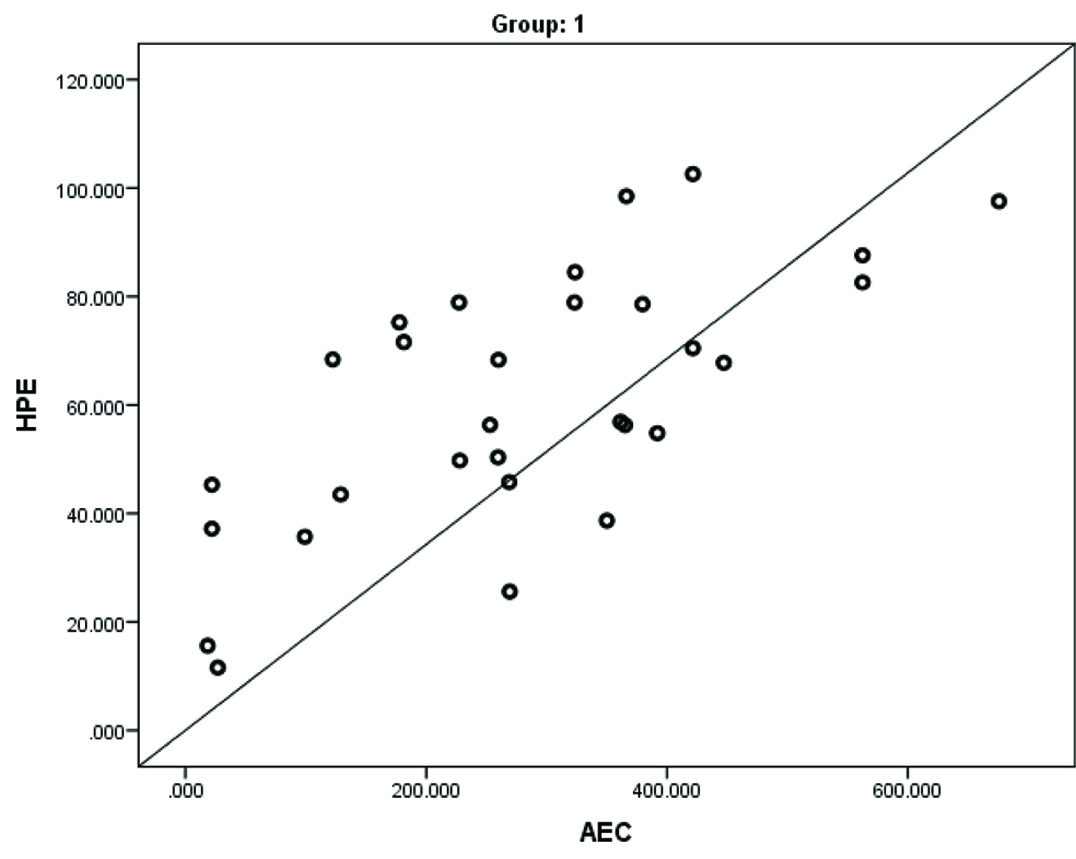
Correlation of Absolute Eosinophil Count (AEC) and histopathology eosinophil count in Group 2.
Discussion
Anatomic variations, infection, genetic susceptibility, and local immunologic imbalance plays main role in CRS [9]. It is suggested that, blood eosinophilia and the extent of eosinophilic inflammation is related to the extent of sinonasal mucosal involvement, the severity of nasal disease, and size of nasal polyps suggesting that CRS with and without NPs represent two end of a spectrum of chronic inflammatory disease [10]. Differences in the expression of inflammatory mediators and cellular characteristics have been demonstrated in CRS and NP mucosal tissue. Data suggests that, eosinophils and related inflammatory products are the hallmark of NP-associated inflammation [9]. However, there is scanty data linking this relationship especially in India.
In the present study, among the patients with Group 1 the male to female ratio was found to be 1.14:1 with 53.3% of the males and in Group 2 the same were noted as 1.75:1 and 63.6% respectively. Similar findings were reported in a study from China by Hu Y et al., [15]. The findings of clinical features in our study were consistent with a recent study by Sakuma Y et al., on 124 patients who reported that asthma complications were significantly different between ECRS and non-ECRS [16]. Study showed that, the new clinical diagnostic criteria for eosinophilic CRS is mainly the increased blood eosinophil counts above the normal range.
In the present study though 82.9% of the patients had abnormal CT findings in osteomeatal complex among the patients with ECRS, a comparable subset of patients had similar findings in non-ECRS (76.7%) group and the difference was statistically not significant (p=0.325). In contrast, Sakuma Y et al., reported that, along with asthma complications, CT image scores were significantly different between ECRS and non-ECRS [16]. In particular, CT image scores for the posterior ethmoid and the olfactory cleft showed good accuracy as predictors of ECRS. A combination of the cut-off values for olfactory cleft score ≥1 and posterior ethmoid score ≥1 indicated high accurate diagnostic ability (sensitivity, 84.6%; specificity, 92.3%).
In our study, significantly higher mean EC (3.6±1.90 vs 10.9±3.80; p<0.001), AEC (283.9±167.00 vs 964.2±729.30; p<0.001) and HPE (61.2±23.70 vs 103.10±60.60; p=0.001) levels were noted in Group 2 compared to Group 1. There was a strong positive linear correlation between AEC and HPE in Group 1 (r=0.686; p<0.001) and 2 (r=0.674; p<0.001). These findings were consistent with the previous literature which showed that patients with ECRS with nasal polyps had higher EC, AEC and HPE levels [13,15–19].
Another recent restrospective study by Hu Y et al., in China found higher peripheral blood eosinophil absolute count and percentage levels in eosinophilic CRSwNP compared with noneosinophilic CRSwNP [15]. Peripheral eosinophil absolute count and percentage were independently and significantly associated with eosinophilic CRSwNP. An absolute blood eosinophil count ≥ 0.215 × 109/L yielded a sensitivity of 74.2% and a specificity of 86.5%, and a blood eosinophil percentage ≥ 3.05% yielded a sensitivity of 80.3% and a specificity of 75.3% for the diagnosis of eosinophilic CRSwNP in the Tongji cohort study [15]. Authors concluded that, eosinophilic and non eosinophilic CRSwNP display significant differences in certain clinical features and peripheral blood eosinophil count could distinguish eosinophilic and noneosinophilic CRSwNP.
A study by Cao PP et al., in China based on the distinct immunopathologic characteristics of various types of CRS in adult Chinese reported that there is increase in number of inflammatory, mononuclear and eosinophil cells in histology specimen of eosinophilic CRSwNP compared to non eosinophilic CRSsNP but there was a decrease in number of mucosal glands [17].
Another similar study by Kim JW et al., demonstrated that the pathogenesis of non eosinophilic nasal polyp may differ from that of eosinophilic nasal polyp considering that the non eosinophilic CRSwNP have thinner basement membrane and have lower number of eosinophils in the peripheral blood compared to eosinophilic nasal polyp [18]. Since other studies have indicated that nasal polyps with high eosinophil count show more aggressive clinical features and tend to recur after medical or surgical treatment [15,16,18,19], this study has shown a simple blood test “blood eosinophil count” to be a good diagnostic and prognostic indicator in deciding close follow up of these patients to avoid reccurence.
It is recommended that blood eosinophil percentage, absolute eosinophil count and MDCT PNS without contrast should be incorporated in the routine preoperative work up of patients with CRS with extensive nasal polyposis and an intraoperative biopsy of the polyp should be send for HPE. Tissue eosinophilia has less improvement of symptoms, quality of life and relapse after endoscopic sinus surgery [14]. In cases with elevated blood eosinophil count (>7%) and tissue eosinophilia (>10 per HPF), it must be kept in mind that steroid nasal wash (budesonide (1 mg) or betamethasone (1 mg in 240 mL of normal saline solution) must be started in the postoperative period as majority of studies show that reccurence rate is less with early initiation of steroid nasal wash [14].
Limitation
Limitation of the study was tissue eosinophilia was not calculated by experienced pathologist but done by authors under guidance of pathologist.
Conclusion
The study demonstrated that there was a strong correlation between tissue and blood eosinophil counts and significantly more severe symptoms are observed in the Eosinophilic CRS with nasal polyps in the Indian population which has implied the severity of the disease in CRS patients with raised eosinophil counts and may affect its management. ECRS with nasal polyp tend to be very aggressive and chances for reccurence is very high post medical/surgical treatment.
Mean Eosinophil count (EC), Absolute Eosinophil count (AEC) and Histopathology Eosinophil Count (HPE) were significantly high in Group 2 compared to Group 1