Dissimilar Pain of Primary Epiploic Appendagitis and Malabsorption
Wolfgang J Schnedl1, Dietmar Enko2, Sandra J Wallner-Liebmann3, Sonja Lackner4, Harald Mangge5
1 Professor, Practice for General Internal Medicine, Bruck, Styria, Austria.
2 Resident, Institute of Laboratory Medicine, Steyr, Upper Austria, Austria.
3 Associate Professor, Institute of Pathophysiology, Centre for Molecular Medicine, Medical University Graz, Graz, Styria, Austria.
4 Research Assistant, Institute of Pathophysiology, Centre for Molecular Medicine, Medical University Graz, Graz, Styria, Austria.
5 Professor, Clinical Institute of Medical and Laboratory Diagnostics, Medical University of Graz, Graz, Styria, Austria.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Wolfgang J Schnedl, Theodor Koernerstrasse 19b, 8600 Bruck, Styria, Austria.
E-mail: w.schnedl@dr-schnedl.at
Primary Epiploic Appendagitis (PEA) is a rare cause of acute or subacute abdominal complaints and non-migratory pain. Usually the diagnosis of PEA is made when Computed Tomography (CT) reveals characteristic figures. Nonspecific abdominal complaints including diffuse abdominal pain may be caused by carbohydrate and/or protein malabsorption. We report a case of a patient with PEA who recovered without medication or surgical treatment within a few days. Eight months later, he was diagnosed with lactose- and histamine malabsorption and Helicobacter pylori infection. The malabsorption was treated successfully with an individually-tailored diet free of culprit triggers and the Helicobacter pylori infection was eradicated. A localized non-migratory abdominal pain caused by PEA needs to be differentiated from nonspecific abdominal complaints due to malabsorption and Helicobacter pylori infection.
Abdominal pain, Computed tomography, Diamine oxidase, Histamine, Lactose
Case Report
A 35-year-old male Caucasian patient with an already existing diagnosis of primary epiploic appendagitis, including images of computed abdominal tomography from 8 months before [Table/Fig-1a,b], presented with continuing nonspecific abdominal complaints. At this presentation, his nonspecific abdominal complaints included postprandial bloating, frequent bowel movements, diffuse abdominal pain and semisolid stools. The physical examination revealed a meteoristic abdomen. Since the characteristics of the abdominal pain changed from a non-migrating dull constant pain in the left lower abdominal quadrant with primary epiploic appendagitis to a diffuse migrating pain mainly in the upper abdomen combined with postprandial bloating, we were reluctant to perform a computed tomography. The patient refused to undergo a gastroscopy but a serum test for Helicobacter pylori using an enzyme-linked immunosorbent assay (Serion ELISA classic, Helicobacter pylori IgA, Würzburg, Germany) demonstrated 49U/ml (normal <20). Using a radio extraction assay for the determination of Diamine Oxidase (DAO) (Sciotec Diagnostic Technologies, Tulln, Austria) the patient was diagnosed with histamine malabsorption when the DAO value was 7.1U/ml (normal >10U/ml) combined with more than 2 nonspecific abdominal symptoms. Additionally hydrogen breath tests were performed for lactose- and fructose intolerance (Gastrolyzer, Bedfont Scientific Inc., Kent, England). During a breath test with a drink containing 50 g lactose load the exhalation of H2 was measured every 30min for a period of 150 min. This test demonstrated an increasing H2-value from baseline 8 up to 29 parts per million (ppm) (normal increase: <20ppm), and during the test bloating and increased bowel movements occurred. Lactose malabsorption was diagnosed. In the breath test with a drink containing 25g fructose load the exhalation of H2 was <20 ppm and fructose malabsorption was not found. Antibodies against tissue transglutaminase were not found, confirming the absence of celiac disease.
Longitudinal (a) and transverse (b) abdominal CT with contrast enhancement, demonstrating primary epiploic appendagitis adjacent to the sigmoid colon in the patient 8 months prior to presentation.
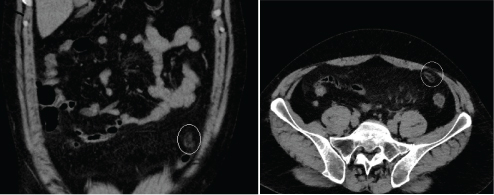
Eight months earlier at the time of diagnosis of Primary Epiploic Appendagitis (PEA) the patient presented with an acute, dull, constant, localized, non-migratory pain in the left lower abdominal quadrant. An abdominal Computed Tomography (CT) with intravenous contrast medium demonstrated an oval lesion, maximum diameter 2.9cm, with a hyperattenuated rim, located adjacent to the sigmoid colon. The recognition of the inflamed and thickened visceral peritoneum surrounding the fat-containing appendage led to the diagnosis of PEA [Table/Fig-1a,b]. Laboratory parameters at the time of diagnosis of PEA were leukocytes 11.7 (normal 4-10x109/L). C-reactive protein and all the other routine laboratory parameters, including erythrocyte sedimentation rate and liver- and pancreas enzymes, were within normal limits.
Within 3 weeks after diagnosis of PEA the patient’s localized pain caused by PEA resolved without therapy. After 8 months, continuing and worsening nonspecific abdominal postprandial complaints occurred repeatedly. To treat the Helicobacter pylori positive gastritis, we suggested a French triple therapy with pantoprazole, clarithromycin and amoxicillin. A registered dietician developed an individually-tailored diet and this dietary intervention resulted in the improvement of symptoms within a few days. Further recovery was uneventful and after six months the patient was still symptom-free. Written informed consent was obtained for all procedures, which were in accordance with the Declaration of Helsinki and the recommendations of the local ethics committee.
Discussion
PEA is an uncommon, benign inflammatory process of epiploic appendages (adipose structures protruding from the colon) and characterized by localized non-migratory abdominal pain [1]. During the last few years, with the increasing use of abdominal CT scans for primary evaluation of acute and subacute abdominal pain, the recognition of PEA has been increasing [2]. Appendices epiploicae are pouches of subserosal fat lining the entire length of the colon. PEA seems to be a localized sterile inflammation in and surrounding one epiploic appendage, and it seems to be primarily caused by a torsion of the appendage with ischemia and infarction, causing aseptic fat necrosis and venous thrombosis. Due to the lack of pathognomonic clinical features and awareness, the diagnosis of PEA is rare, but CT is the diagnostic modality of choice [3]. This acute and subacute abdominal pain is dull, constant and non-migrating, and physical examination reveals a well-localized area of tenderness. Most cases of PEA are located at the sigmoid colon and it was speculated that patients with mild diverticulitis might have PEA. However, it was suggested that even up to 7% of all patients clinically suspected of having diverticulitis may have PEA [4]. The dull, constant, non-migrating, localized, acute and subacute abdominal pain is characteristic of PEA and is reported in most patients with PEA. We agree with the suggestion of a 30% prevalence of malabsorption in PEA [5], although nonspecific abdominal complaints usually do not occur in PEA.
Malabsorption syndromes are caused by carbohydrates or proteins e.g., fructose, gluten, histamine, and lactose. Food components are not absorbed and digested properly during gastrointestinal passage in these disease conditions. This results in symptoms due to bacterial metabolism and fermentation in the colon. Malabsorption syndromes caused by carbohydrates and proteins are frequently observed in patients with nonspecific abdominal complaints, which include postprandial fullness, flatulence, bloating, belching, irregular bowel movements, cramps and pain, loose stools, diarrhea, and obstipation usually linked to ingestion of food containing triggers of malabsorption [6]. Malabsorption is frequent in patients with nonspecific gastrointestinal complaints and can cause gastrointestinal symptoms mimicking Irritable Bowel Syndrome (IBS). Various combinations of malabsorption may occur and are increasingly reported in patients with malabsorption syndromes [7,8].
Lactose intolerance is related to lactase deficiency and causes nonspecific gastrointestinal complaints with the ingestion of dairy products. Histamine intolerance is a disproportionate amount of histamine in the body caused by the consumption of histamine-containing food or drinks, and/or a reduced ability of enzymes to digest histamine. Within the gastrointestinal tract DAO seems to be the primary enzyme for the digestion of histamine. Due to the variability of symptoms observed in multiple organs, the diagnosis of histamine intolerance is generally difficult. In this patient the diagnosis of histamine intolerance was made with a diamine oxidase value <10U/ml in serum and more than 2 typical symptoms belonging to nonspecific abdominal complaints [9]. The gram-negative pathogen in the stomach Helicobacter pylori influence the microbiota composition in the GI tract and are discussed to cause a change in the intestinal microorganisms leading to nonspecific abdominal complaints [10]. Eradication of Helicobacter pylori was performed in this patient and this contributed to the improvement of his symptoms.
A diet free of triggering food components and eradication of Helicobacter pylori infection leads to the improvement of nonspecific abdominal complaints in a high percentage of patients. Since in this patient these nonspecific abdominal symptoms were present for months, they appear not to be linked to the acute localized abdominal pain due to PEA. Therefore, we tested this patient for malabsorption and Helicobacter pylori infection and found a combination of lactose and histamine malabsorption as well as a Helicobacter pylori infection. However, this patient with nonspecific abdominal complaints due to lactose and histamine malabsorption recovered with a diet free of symptom-causing triggers and Helicobacter pylori eradication therapy within a few days.
Recognition of a characteristic lesion on CT scans with acute/subacute non-migratory abdominal pain may lead to the diagnosis of PEA, and outpatient treatment without any medication or surgery is successful within a few days. However, nonspecific abdominal symptoms may be due to carbohydrate and/or protein malabsorption and can be treated effectively with an individually-tailored diet free of culprit triggers [11].
Declarations
Competing interests Wolfgang J. Schnedl is co-founder of GedoMed GmbH. The other authors declare no competing interests.
Ethical approval: Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Conclusion
In conclusion, we state that an abdominal localized non-migratory pain caused by PEA needs to be differentiated from nonspecific abdominal complaints due to malabsorption syndromes and Helicobacter pylori infection.
[1]. Schnedl WJ, Krause R, Tafeit E, Tillich M, Lipp RW, Wallner-Liebmann SJ, Insights into epiploic appendagitisNat Rev Gastroenterol Hepatol 2011 8:45-49. [Google Scholar]
[2]. Ozdemir S, Gulpinar K, Leventoglu S, Uslu HY, Turkoz E, Ozcay N, Torsion of the primary epiploic appendagitis: A case series and review of literatureAm J Surg 2010 199:453-58. [Google Scholar]
[3]. Singh AK, Gervais DA, Hahn PF, Rhea J, Mueller PR, CT appearance of acute epiploic appendagitisAm J Roentgenol 2004 183:1303-07. [Google Scholar]
[4]. Hwang JA, Kim SM, Song HJ, Lee YM, Moon KM, Moon CG, Differential diagnosis of left-sided abdominal pain: Primary epiploic appendagitis vs colonic diverticulitisWorld J Gastroenterol 2013 19:6842-48. [Google Scholar]
[5]. Schnedl WJ, Lipp RW, Wallner-Liebmann SJ, Kalmar P, Szolar DH, Mangge H, Primary epiploic appendagitis and fructose malabsorptionEur J Clin Nutr 2014 68:1359-61. [Google Scholar]
[6]. Lomer MCE, Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intoleranceAliment Pharmacol Ther 2014 41:262-75. [Google Scholar]
[7]. Goebel-Stengel M, Stengel A, Schmidtmann M, van der Voort I, Kobelt P, Mönnikes H, Unclear abdominal discomfort: Pivotal role of carbohydrate malabsorptionJ Neurogastroenterol Motil 2014 20:228-35. [Google Scholar]
[8]. Enko D, Kriegshäuser G, Halwachs-Baumann G, Mangge H, Schnedl WJ, Serum diamine oxidase activity is associated with lactose malabsorption phenotypic variationClin Biochem 2016, in press [Google Scholar]
[9]. Maintz L, Novak N, Histamine and histamine intoleranceAm J Clin Nutr 2007 85:1185-96. [Google Scholar]
[10]. Ierardi E, Goni E, Losurdo G, Di Mario F, Helicobacter pylori and non-malignant diseasesHelicobacter 2014 19(Suppl. 1):27-31. [Google Scholar]
[11]. Kohn JB, Is there a diet for histamine intolerance?J Acad Nutr Diet 2014 114:1860 [Google Scholar]