Assessment of Autonomic Dysfunction in Acute Stroke Patients at a Tertiary Care Hospital
Hemachandrika Chidambaram1, Kothai Gnanamoorthy2, Prasanna Karthik Suthakaran3, Kannan Rajendran4, Chitrambalam Pavadai5
1 Professor, Department of Physiology, Kilpauk Medical College, Kilpauk, Chennai, Tamilnadu, India.
2 Assistant Professor, Department of General Medicine, SRM Medical College Hospital and Research Centre, Potheri, Chennai, Tamilnadu, India.
3 Associate Professor, Department of General Medicine, Saveetha Medical College Hospital, Thandalam, Chennai, Tamilnadu, India.
4 Professor, Department of General Medicine, Saveetha Medical College Hospital, Thandalam, Chennai, Tamilnadu, India.
5 Professor, Department of General Medicine, Saveetha Medical College Hospital, Thandalam, Chennai, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Prasanna Karthik, New No 16, Third Street, Bakthavatsalam Nagar, Adyar, Chennai-600020, Tamilnadu, India.
E-mail: kartpress@gmail.com
Introduction
In patients who present with acute cerebro-vascular disease, autonomic function testing is usually not given its due importance. This is because of the complex nature of the autonomic function tests and the relative technical difficulty faced in administering the tests to the patients. A simple and non-invasive method to assess the autonomic dysfunction is measurement of resting Heart Rate Variability (HRV).
Aim
To study the pattern of autonomic dysfunction among patients admitted with acute stroke and to study the relationship between autonomic dysfunction and the morbidity and mortality associated with acute stroke.
Materials and Methods
The study was carried out on 97 patients who were admitted with diagnosis of acute stroke. Patients with conduction abnormalities on ECG were excluded from the study. Resting ECG tracings were obtained for a period of 5 minutes. The frequency domain analysis of HRV was performed by a Fast Fourier transform of the RR intervals. The High Frequency (HF) was representative of the parasympathetic activity while low frequency is representative of baroreceptor mediated parasympathetic and sympathetic activity and Low Frequency (LF)/HF ratio was a measure of the sympathovagal balance. Statistical analysis was carried out with student’s t-test and chi-square test and p-value ≥ 0.05 was taken to be statistically significant.
Results
The mean age of the patients was 60.84±14.12 years. A total of 41 patients were females and 77 patients had ischemic stroke. Out of the total 97, 60 patients had evidence suggestive of increased sympathetic activity with a mean LF/HF ratio of 2.03±0.88. These patients had significantly higher mean systolic BP, diastolic BP and National Institute of Health Stroke Scale (NIHSS) values when compared to patients with reduced LF/HF ratio (166.33±24.81 vs 148.54±19.42, p=0.0003, 100.33±18.73 vs 88.76±12.66, p=0.0013, 15.77±8.22 vs 11.49±6.63, p=0.0088 respectively). These patients also had a higher mortality rate.
Conclusion
This study highlights the problem of autonomic dysfunction among patients with stroke. Patients with autonomic dysfunction had higher morbidity and mortality in the acute phase of stroke in this study and also had higher blood pressure readings. This is a small scale study whose findings need to be validated further by larger population studies.
Autonomic function tests, Cerebrovascular disease, Heart rate variability, Sympathovagal balance
Introduction
Cerebrovascular diseases or stroke is defined as an abrupt onset of a neurologic deficit that is attributable to a focal vascular cause [1]. The diagnosis of stroke is made on clinical grounds and imaging studies are used to support and confirm the diagnosis. The clinical manifestations of stroke are myriad due to the complex relationship between the regions of the brain and the cerebral vasculature. Of late, it has emerged as one of the leading contributors to the morbidity and mortality of our nation’s population [2]. Although it has a specific age predilection, in that being common among the elderly, it does not spare the young too in whom the effects are more dramatic.
The patients who present with acute cerebrovascular disease are traditionally evaluated to determine the extent of the involvement of the higher mental functions, cranial nerves, motor system, sensory system, extrapyramidal system, cerebellar functions and autonomic functions. Autonomic function testing is usually not given its due importance. This is because of the complex nature of the autonomic function tests and the relative technical difficulty faced in administering the tests to the patients. A certain level of cooperation from the patient and a degree of mobility of the patient is definitely required to carry out the battery of autonomic function tests [3] [Table/Fig-1].
Ewing and Clarke’s autonomic function tests.
| Test | Measure | Method |
|---|
| Valsalvamaneuver | Valsalvaratio | The subject sits quietly and then blows into a mouthpiece at a pressure of 40 mmHg for 15s. The ratio of the longest R-R interval shortly after the maneuver to the shortest R-R interval during the maneuver is calculated |
| Lying tostanding heartrate response | 30:15 ratio | The subject lies quietly on a couch and then stands up unaided. The 30:15 ratio is the ratio of the longest R-R interval around the 30th beat to the shortest R-R interval around the 15th beat after standing up |
| Heart rateresponse todeep breathing | Max – Minheart rate(beats/min) | The subject sits quietly and then breathes deeply and evenly at 6 breaths/min. The maximum and minimum heart rates during each breathing cycle are measured |
| Postural blood pressure change | Fall insystolicBP (mmHg) | The blood pressure is measured while the subject is lying down, and again after standing up. The difference in systolic BP is calculated |
| Sustainedhandgriptest | Rise indiastolicBP (mmHg) | Handgrip is maintained at 30% of the maximum voluntary contraction using a handgrip dynamometer up to a maximum of 5 min. The difference between the diastolic BP just before release of handgrip, and before starting, is calculated |
As this is not usually possible in these bed ridden acutely ill stroke patients, a simpler method to assess the autonomic dysfunction becomes mandatory. This role is well fulfilled by the simple and non-invasive measurement of resting HRV. According to Sztajzel J., among the various techniques available for assessing the autonomic status, HRV is a good tool to evaluate the sympathovagal balance at the sinoatrial level [4].
The problem of autonomic dysfunction in patients with acute cerebrovascular diseases is still not given adequate importance as the mechanisms behind such dysfunction are still unclear. Sykora M. et al., have highlighted the potential therapeutic aspects of baroreflex in patients with acute stroke [5].
The effect of cardiac autonomic dysfunction on mortality and morbidity of patients with diabetes and acute myocardial infarction have been well established and the benefits of targeting the HRV by multiple modalities of treatment have improved outcomes in these patients [6]. So, the presence of autonomic dysfunction may play a role in the development of cardiac arrhythmias in patients with stroke and interventions directed towards reducing the incidence of it may play a role albeit in decreasing the morbidity as well as mortality associated with cerebrovascular diseases. However, the use of different treatment approaches to treat the autonomic dysfunction associated with ischemic stroke has led to varied results [7].
In this regard, there is an urgent need for data on autonomic dysfunction in patients with stroke from India. There has been very few studies which have been able to document the prevalence of autonomic dysfunction among patients with first ever stroke and its clinical outcome. This study was designed to address the gap in this knowledge and aimed at documenting the pattern of autonomic dysfunction in patients with acute stroke.
Materials and Methods
The present study was designed as a prospective, open-label study carried out on patients who were admitted to a tertiary care referral hospital. The protocol of the study was approved by the Institutional Ethics Committee and Institutional Scientific Research Board prior to the commencement of the study. Detailed informed written consent was obtained prior to enrolment in the study from either the patient (if possible) or else from the legal guardian of the patient. The study was carried out for a period of 6 months (November 2015 – May 2016) and all patients who were brought to the hospital were deemed to be eligible for the study if they fulfilled the inclusion and exclusion criteria.
Patients aged above 18 years of either sex were enrolled for the study if they met the inclusion criteria for first ever episode of acute stroke, which was defined as onset of the focal neurological symptoms less than 7 days prior to admission and confirmed by clinical examination of neurological deficit and/or radiological imaging evidence of cerebrovascular disease. Patients with previous history of stroke, heart disease and patients who were on medications known to affect the conduction system of the heart were excluded from the study. A baseline ECG at the time of admission was done to rule out any conduction abnormalities. Patients with such defects were excluded from the study as the software interpretation of such patients has not yet been validated.
The severity of the stroke was established using the National Institute of Health Stroke Scale [8]. The scale uses 11 different items to assess the patient and each item rated on a scale ranging between 0 and 4 and the individual scores are added. The maximum possible score is 42. The score 0 usually represents no limitation of the ability while higher scores reflect significant limitation in the ability. The findings from a relevant radiological investigation were used for determining the type of stroke the patient had (ischemic or haemorrhagic). The patients were followed up to 30 days and their survival was noted.
ECG tracings {using NIVIQURE Ambulatory Digital ECG Recorder (INCO)} were obtained after the patients were allowed to rest in the supine position for five minutes in a calm and isolated room prior to initiation of ECG recording. The sampling rate was set to 1024 Hz and the recording interval was set to 320 sec (5min = 300 sec with 10 sec lead in and 10 sec lead out period). The recorded data was screened for the presence of artefacts and appropriately edited according to guidelines to ensure the validity of the result [9]. The edited data was analysed with the available software (Heart Rate Variability Analysis Software version 1.1, Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland). The frequency domain analysis of HRV was performed by a Fast Fourier transform of the RR intervals, which produced a power spectrum from the 0.01-Hz to 1.0-Hz unit. Three frequency domain measures of HRV namely LF (range, 0.04 to 0.15 Hz), HF (range, 0.15 to 0.40 Hz), and LF/HF ratio, were calculated. The LF is representative of baroreceptor mediated parasympathetic and sympathetic activity, HF is representative of the parasympathetic activity while LF/HF ratio is a measure of the sympathovagal balance. The LF/HF ratio more than 1 indicates the presence of increased sympathetic discharges in the body.
Statistical Analysis
Statistical analysis was carried out with the help of Graphpad Prism 6 software using unpaired student’s t-test for parametric and chi-square test for non-parametric data. A p-value ≥ 0.05 was taken to be statistically significant.
Results
A total of 97 patients were enrolled in the study. The mean age of the patients was 60.84±14.12 years. Out of the 97 patients, 42.26% (n=41) patients were females. 79.38% (n=77) patients had ischemic stroke while the remaining 20.62% patients had haemorrhagic stroke [Table/Fig-2].
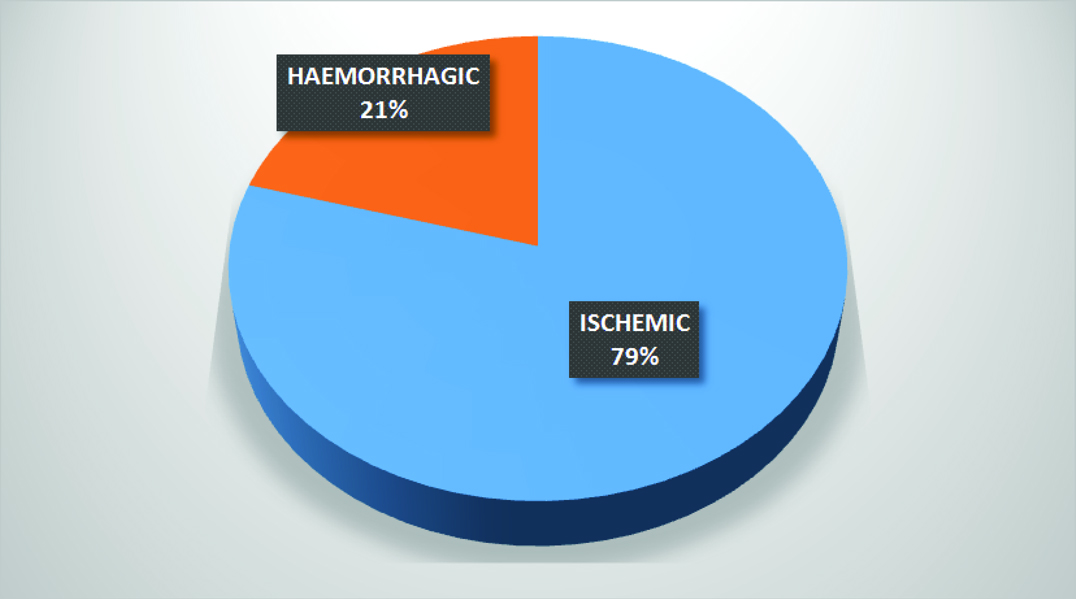
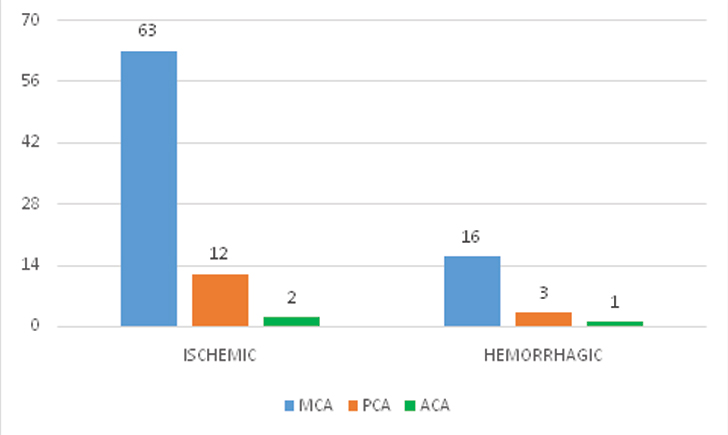
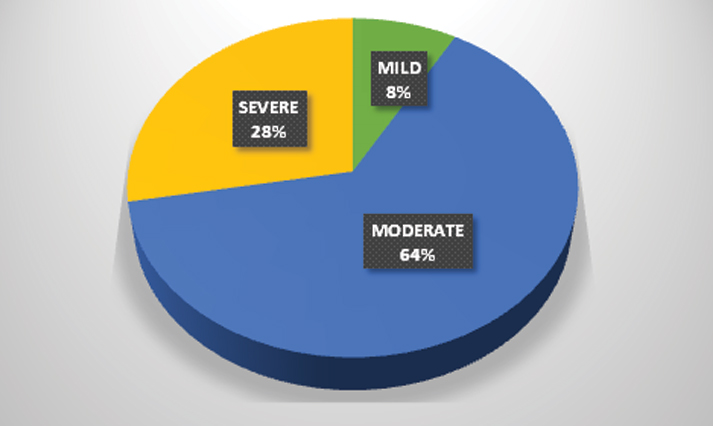
The majority of patients (81.82%, n = 63) with ischemic stroke had involvement of the middle cerebral artery while anterior cerebral artery was involved only in 2.6% (n = 2) of the patients [Table/Fig-3]. This was similar in the haemorrhagic group as well where 80% of patients (n=16) had involvement of the middle cerebral artery territory. The overall fatality at 30 days was 29.89% (n= 29). The distribution of NIHSS scores is given in [Table/Fig-4].
Arterial territory involved in ischemic and haemorrhagic stroke.
MCA- Middle Cerebral Artery, PCA- Posterior Cerebral Artery, ACA- Anterior Cerebral Artery
Distribution of NIHSS scores at the time of admission.
The patients were divided into two sub groups on the basis of the LF/HF ratio. Group 1 had patients with LF/HF > 1 while group 2 had patients with LF/HF < 1. The various parameters are described in [Table/Fig-5].
Baseline characteristics and autonomic function tests.
| GROUP 1LF/HF > 1(n = 60) | GROUP 2LF/HF < 1(n = 37) | p-value |
|---|
| Age (years) | 63.10±13.68 | 57.59±13.85 | 0.0583NS |
| Females /Males | 24/36 | 18/19 | 0.5272NS |
| SBP (mm Hg) | 166.33±24.81 | 148.54±19.42 | 0.0003* |
| DBP (mm Hg) | 100.33±18.73 | 88.76±12.66 | 0.0013* |
| NIHSS score | 15.77±8.22 | 11.49±6.63 | 0.0088* |
| LF power (nu) | 63.4362±8.8853 | 31.9576±10.5947 | < 0.0001** |
| HF power (nu) | 34.9103±8.9349 | 66.3546±10.1179 | < 0.0001** |
| LF/HF ratio | 2.029±0.87712 | 0.51762±0.24648 | < 0.0001** |
| Survival | 37/60 | 31/37 | 0.0237* |
Results expressed as Mean±SD, NS – Not significant, * - Significant, ** - Highly significant
Discussion
The mean age of patients in the present study was 60.84 years which was similar to the findings reported from Mumbai (66 years) and Trivandrum (67 years) stroke registries while slightly higher than the data from Bangalore (54.5 years) registry [10–12]. Males were commonly affected by stroke in this study which was similar to the data from Mumbai and Bangalore while different from the Trivandrum data which found females to be affected more. Ischemic stroke accounted for nearly 80% of the patients which is similar to data reported from the Mumbai (80.2%) and Trivandrum (83.6%) stroke registries. The overall fatality rate was about 30% which is about the same as data from Mumbai (29.8%) and Trivandrum (27.2%) registries [10,11]. In the present study, 8.24% (n = 8) of patients had minor stroke while 27.84% (n=27) patients had severe stroke according to the NIHSS score at the time of admission.
A total of 60 patients out of the total 97 had evidence suggestive of increased sympathetic activity as shown by their increased LF/HF ratio. This is similar to the findings of Tokgozoglu et al., and Colivicchi et al., who found that, patients with acute stroke had elevated values of LF/HF ratio when compared to the normal population [13,14]. The patients with higher LF/HF ratio had significantly higher mean systolic and diastolic BP-values when compared to patients with reduced LF/HF ratio (166.33±24.81 vs 148.54±19.42, p=0.0003, 100.33±18.73 vs 88.76±12.66, p=0.0013). This was similar to findings established by Hilz et al., who showed that in patients with MCA territory stroke elevated LF/HF values were associated with elevated systolic and diastolic blood pressures. They also showed that, in acute ischemic stroke patients the NIHSS correlated significantly with normalized LF/HF-ratios. In this study, the mean NIHSS score was higher in patients with LF/HF ratio > 1 and this difference was statistically significant [15]. Makikallio et al., however reported no elevations in LF/HF ratio in patients with increasing stroke severity [16].
In this study, elevated levels of LF/HF ratio were associated with a statistically significant decrease in survival. In the study by Tokgozoglu et al., the LF/HF ratio was found to be higher in patients who died suddenly after ischemic stroke when compared with survivors, though this difference was not found to be statistically significant [13]. Multiple studies have documented the impact of autonomic dysfunction and HRV in both the short term and long term survival of patients with ischemic stroke [17–19].
In a review article, Yperzeele et al., concluded that, the assessment of autonomic dysfunction may play a diagnostic and prognostic role in patients with acute stroke and that a short-term HRV analysis, performed upon patients’ arrival at the emergency department or even in the prehospital phase, could lead to predictions on outcome and potential complications [20].
Limitation
This study was carried out in a relatively small population. Hence, there is a need to confirm the findings from larger population studies which will also provide normative population data for Indian patients having autonomic dysfunction. Further studies will also potentially help overcome limitations in the current models of our understanding of the functioning of the autonomic system which hamper our ability to understand the impact of cerebrovascular diseases on the autonomic dysfunction that occurs post stroke.
Conclusion
This study highlights the presence of autonomic dysfunction among patients with stroke from a tertiary care hospital. Patients with autonomic dysfunction had higher morbidity and mortality in the acute phase of stroke in this study and also had higher blood pressure readings. There was no age or sex predilection for autonomic dysfunction.
Results expressed as Mean±SD, NS – Not significant, * - Significant, ** - Highly significant
[1]. Smith WS, Johnston SC, Hemphill JC, Cerebrovascular Diseases. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J edsHarrison’s Principle of Internal Medicine 2015 19th Edsl.McGraw Hill Education:2559-86. [Google Scholar]
[2]. Pandian JD, Sudhan P, Stroke epidemiology and stroke care services in IndiaJ Stroke 2013 15(3):128-34. [Google Scholar]
[3]. Ewing DJ, Martyn CN, Young RJ, Clarke BF, The value of cardiovascular autonomic function tests: 10 years’ experience in diabetesDiabetes Care 1985 8:491-98. [Google Scholar]
[4]. Sztajzel J, Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous systemSwiss Med Wkly 2004 134:514-22. [Google Scholar]
[5]. Sykora M, Diedler J, Turcani P, Hacke W, Steiner T, Baroreflex: A new therapeutic target in human stroke?Stroke 2009 40:e678-82. [Google Scholar]
[6]. Balcıoglu AS, Müderrisoglu H, Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatmentWorld J Diabetes 2015 6(1):80-91. [Google Scholar]
[7]. De Raedt S, De Vos A, De Keyser J, Autonomic dysfunction in acute ischemic stroke: An underexplored therapeutic area?J Neurol Sci 2015 348:24-34. [Google Scholar]
[8]. National Institute of Health, National Institute of Neurological Disorders and StrokeStroke Scalehttp://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf [Google Scholar]
[9]. Task Force of the European Society of Cardiology and The North American Society of pacing and electrophysiology. Heart rate variability; standards of measurements, physiological interpretation, and clinical useEur Heart J 1996 17:354-81. [Google Scholar]
[10]. Dalal PM, Malik S, Bhattacharjee M, Trivedi ND, Vairale J, Bhat P, Population-based stroke survey in Mumbai, India: Incidence and 28-day case fatalityNeuroepidemiology 2008 31:254-61. [Google Scholar]
[11]. Sridharan SE, Unnikrishnan JP, Sukumaran S, Sylaja PN, Nayak SD, Sarma PS, Incidence, types, risk factors, and outcome of stroke in a developing country: The Trivandrum Stroke RegistryStroke 2009 40:1212-18. [Google Scholar]
[12]. Nagaraja D, Gururaj G, Girish N, Panda S, Roy AK, Sarma GR, Feasibility study of stroke surveillance: data from Bangalore, IndiaInd J Med Res 2009 130(4):396-403. [Google Scholar]
[13]. Tokgozoglu SL, Batur MK, Topcuoglu MA, Saribas O, Kes S, Oto A, Effects of stroke localization on cardiac autonomic balance and sudden deathStroke 1999 30:1307-11. [Google Scholar]
[14]. Colivicchi F, Bassi A, Santini M, Caltagirone C, Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvementStroke 2004 35:2094-98. [Google Scholar]
[15]. Hilz MJ, Moeller S, Akhundova A, Marthol H, Pauli E, De Fina P, High NIHSS values predict impairment of cardiovascular autonomic controlStroke 2011 42:1528-33. [Google Scholar]
[16]. Makikallio AM, Makikallio TH, Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllyla VV, Heart rate dynamics predict poststroke mortalityNeurology 2004 62:1822-26. [Google Scholar]
[17]. Chen PL, Kuo TB, Yang CC, Parasympathetic activity correlates with early outcome in patients with large artery atherosclerotic strokeJ Neurol Sci 2012 314:57-61. [Google Scholar]
[18]. Sykora M, Steiner T, Rocco A, Turcani P, Hacke W, Diedler J, Baroreflex sensitivity to predict malignant middle cerebral artery infarctionStroke 2012 43:714-19. [Google Scholar]
[19]. Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF, Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic strokeStroke 2003 34:705-12. [Google Scholar]
[20]. Yperzeele L, van Hooff RJ, Nagels G, De Smedt A, De Keyser J, Brouns R, Heart rate variability and baroreceptor sensitivity in acute stroke: a systematic reviewInt J Stroke 2015 10:796-800. [Google Scholar]