Dasatinib Induced Cardiac Tamponade-A Rare Association
Sushant Wattal1, Mugula Sudhakar Rao2, GS Naveen Chandra3, U.K. Abdul Razak4, K Ranjan Shetty5
1 Registrar, Department of Cardiology, KMC, Manipal, Karnataka, India.
2 Registrar, Department of Cardiology, KMC, Manipal, Karnataka, India.
3 Assistant Professor, Department of Cardiology, KMC, Manipal, Karnataka, India.
4 Assistant Professor, Department of Cardiology, KMC, Manipal, Karnataka, India.
5 Professor, Department of Cardiology, KMC, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Abdul Razak U.K., Assistant Professor, Department of Cardiology, KMC, Manipal-576104, Karnataka, India.
E-mail: msudhakar88@gmail.com
Drug induced cardiac tamponade is rare. Therapy for imatinib resistant Chronic Myeloid Leukaemia (CML) is an emerging challenge in clinical haematology. For such cases treatment with second line tyrosine kinase inhibitors like dasatinib has resulted in improved outcomes. Dasatinib is a second line BCR-ABL tyrosine kinase inhibitor used in the treatment of Imatinib resistant or Imatinib intolerant CML. Dasatinib has been reported to cause severe pericardial effusions in 1% of all patients in clinical studies. We report here a case of Dasatinib induced cardiac tamponade in whom all other causes of pericardial effusion were excluded and whose clinical symptoms as well as effusion showed no recurrence one month after the drug was stopped.
Chronic myeloid leukaemia, Pericardial effusion, Tyrosine kinase inhibitor
Case Report
A 48-year-old male patient presented to the cardiology outpatient department with complaints of shortness of breath for the past one month. The onset was insidious and gradually progressive, on ordinary activity to start with and on less than ordinary activity for the past one week. He was diagnosed as a case of chronic myeloid leukaemia five years prior to current admission and was on treatment with Imatinib mesylate for three years. However, due to the development of resistance and poor treatment response, he was started on Dasatinib 100 mg once daily two years back.
On general physical examination, patient was afebrile and was found to have cold extremities, tachycardia (110/min), tachypnoea (30 breaths/min) and hypotension (BP-90/60 mm Hg). He had pulsus paradoxus and jugular venous pressure was raised with prominent X descent, absent Y descent and showing normal decrease on inspiration. On systemic examination his apical impulse was poorly localised and on auscultation, his heart sounds were muffled. Examination of chest revealed decreased bilateral air entry, but more decreased on the right side.
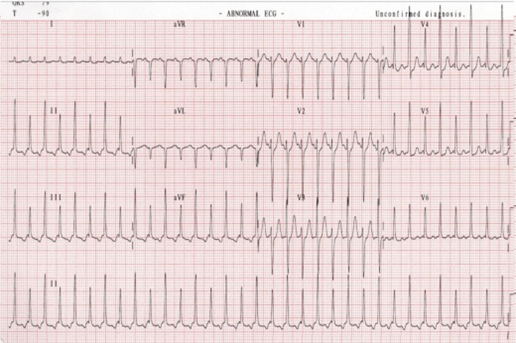
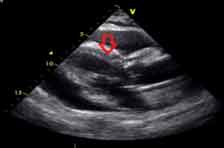
Chest X-ray was suggestive of bilateral pleural effusion, on the right side more than left, with cardiomegaly. Electrocardiography showed electric alternans [Table/Fig-1], while two dimensional echocardiography showed massive pericardial effusion all around the heart, with collapse of right atrium and right ventricle occuring early during diastole [Table/Fig-2]. Inferior vena cava was dilated and showed no size variation with respiration. An exaggerated respiratory variation on Doppler velocity recordings was seen in the trans-valvular flow with inspiratory increases on the right and decreases on the left.
Twelve Lead electrocardiogram showing inverted P waves in inferior leads suggestive of ectopic atrial tachycardia and electrical alternans.
Two Dimensional echocardiogram in parasternal long axis view showing massive pericardial effusion all around the heart along with arrow showing diastolic collapse of right ventricle.
Pericardiocentesis was done and 600 ml of pericardial fluid was removed. Pericardial fluid was subsequently sent for analysis. Patient symptoms were relieved following pericardiocentesis. However, repeat pericardial collection was seen on repeat echocardiogram the next day. Repeat pericardiocentesis of 600 ml was done.
Pericardial fluid analysis showed exudative nature of the fluid with high protein (6.5 g/dl), low glucose content (36 mg/dl) and high LDH level (304 U/L); while the ADA Level was low at only 16 U/L. Pathological analysis showed 190 cells with lymphocytes being the dominant cell type (86%).
No malignant cells were seen on cytological analysis with benign mesothelial cells in a background of neutrophils and lymphocytes dominating the field. Pericardial fluid showed no microbial growth on culture even after 72 hours. Fluid was sent for PCR for Mycobacterium tuberculosis, which turned out to be negative.
Post pericardiocentesis contrast enhanced CT scan of the chest showed mild pericardial effusion and bilateral pleural effusion. Dasatinib was stopped patient was restarted on imatinib mesylate. There was no recurrence of serositis on imatinib and patient was treated with prednisolone. Subsequently repeat echocardiography after 2 weeks showed no significant increase in pericardial fluid collection. This proved that it was a case of Dasatinib induced cardiac tamponade and pleural effusion.
Discussion
BCR-ABL tyrosine kinase plays a key role in the pathogenesis of CML, thus acting as the main target for drugs for its treatment. Imatinib (Gleevec®; Novartis Pharmaceuticals Corporation, New Jersey, USA), despite its remarkable success has to be discontinued in many patients due to either resistance or adverse effects. Therapy for imatinib resistant CML is an emerging challenge in clinical haematology [1–3]. So in such cases increasing imatinib dose, or changing therapy to second line agents like dasatinib {Sprycel®; Bristol-Myers Squibb Co. (BMS), New York, USA} is the treatment of choice.
Dasatinib is a potent inhibitor of BCR-ABL. It has 325-fold greater activity against native BCR-ABL as compared to imatinib. Complete Cytogenetic Responses (CCyR) have been achieved in a large number of imatinib-resistant patients. Pleural effusion is the most frequent non-haematologic adverse effect of dasatinib [4]. It occurs due to serosal inflammation and is more common in advanced disease [5].
Tyrosine kinase inhibitors including Dasatinib are known to have potential cardiotoxic effects according to recent evidences. Cardiac side effects commonly reported are pericardial effusions, arrhythmia, QT prolongation, myocardial ischemia, congestive heart failure and myocardial infarction. Pathogenesis of Dasatinib induced pleural and pericardial effusion may involve inactivation of platelet derived growth factor or expansion of cytotoxic T and natural killer cells. Dasatinib induced serositis presents a significant treatment challenge to physicians, as it cannot be predicted, the time of its onset is variable, and repeat invasive procedures are frequently required for its management. Pre-existing comorbidities, such as cardiac or pulmonary disorders may influence risk of effusion formation [4,5]. Viral infection and an increase in activated lymphocytes (LGL cells) are also reported as potential risk factors for effusion formation [6].
Evidence for Dasatinib induced severe pericardial effusions have been reported in 1% of all patients in all clinical studies and a prescriber alert for Dasatinib is usually issued, warning about this very toxicity [7].
In one case study, out of the 13 CML patients treated with Dasatinib at 100 mg or 50 mg daily, four developed clinically relevant pleural or pericardial effusions. Two of these four patients developed grade III or IV effusions and one was found to have a life-threatening pericardial effusion. Effusion was also seen in one of the two patients who were started on front-line therapy with Dasatinib at 100 mg daily [8].
Management for such effusions caused by Dasatinib is to withhold the drug or reduce the dose until the effusion has resolved or improved as well as supportive therapy and to restart at an appropriate dose, according to the initial severity of event [9,10].
Conclusion
Though rare Dasatinib induced pericardial effusion should always be kept in mind in all patients receiving the drug who develop symptoms suggestive of pericardial effusion. An attempt should be made on stopping the drug and changing to other newer tyrosine kinase inhibitors.
[1]. Martinelli G, Soverini S, Rosti G, Cilloni D, Baccarani M, New tyrosine kinase inhibitors in chronic myeloid leukemiaHaematologica 2005 90(4):534-41. [Google Scholar]
[2]. Valent P, Emerging stem cell concepts for imatinib-resistant CML: implications for the biology, management, and therapy of the diseaseBr J Haematol 2008 142(3):361-78. [Google Scholar]
[3]. Saglio G, Ulisciani S, Bosa M, Cilloni D, Rege-Cambrin G, New therapeutic approaches and prognostic factors in chronic myeloid leukemiaLeuk Lymphoma 2008 49(4):625-28. [Google Scholar]
[4]. Sillaber C, Herrmann H, Bennett K, Rix U, Baumgartner C, Böhm A, Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg dailyEur J Clin Invest 2009 39(12):1098-109. [Google Scholar]
[5]. Kelly K, Swords R, Mahalingam D, Padmanabhan S, Giles FJ, Serosal inflammation (pleural and pericardial effusions) related to tyrosine kinase inhibitorsTarget Oncol 2009 4(2):99-105. [Google Scholar]
[6]. Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapyLeukemia 2009 23(8):1398-405. [Google Scholar]
[7]. Kronzon I, Cohen ML, Winer HE, Cardiac tamponade by loculated pericardial haematoma: limitations of M-mode echocardiographyJ Am Coll Cardiol 1983 1:913-15. [Google Scholar]
[8]. Krauth MT, Herndlhofer S, Schmook MT, Mitterbauer-Hohendanner G, Schlögl E, Valent P, Extensive pleural and pericardial effusion in chronic myeloid leukemia during treatment with Dasatinib at 100 mg or 50 mg dailyHaematologica 2011 96(1):163-66. [Google Scholar]
[9]. Masiello D, Gorospe G, Yang AS, The occurrence and management of fluid retention associated with TKI therapy in CML, with a focus on DasatinibJ Haematol Oncol 2009 2:46 [Google Scholar]
[10]. Mauro MJ, Deininger MW, Management of drug toxicities in chronic myeloid leukaemiaBest Pract Res Clin Haematol 2009 22(3):409-29. [Google Scholar]