There has been a tremendous increase in the incidence of cardiovascular diseases over the past few decades. Patients admitted in ICU are prone to DDI due to complex pharmacotherapy, multiple medications, severity of illness, and organ failure [1–4]. It has been observed that patients presenting with cardiovascular diseases are at an increased risk of DDI due to polypharmacy and the effects of cardiac disease on metabolism of drug [5]. A study conducted previously has shown that adverse drug reactions due to DDIs accounted for about 0.05% of visits to emergency department, 0.6% of hospitalization and about 0.1% of re-admissions in hospital [6]. It is therefore, imperative for the physician to avoid prescribing unnecessary drugs to the patients as most of the DDI are iatrogenic but avoidable. Monitoring of DDI is necessary to minimize the risk of adverse drug reactions and cost of the treatment.
DDIs are observed when effects of one drug are modified by the simultaneous administration of another drug [7]. These interactions can be either pharmacokinetic or pharmacodynamic. Pharmacokinetic interaction is said to occur when one drug affects the effect of other drug by change in absorption, distribution, metabolism or excretion of another drug. On the other hand, pharmacodynamic interaction is seen when the two drugs either exhibit synergism or antagonism in their mechanism of action. There is paucity of data highlighting potential drug-drug interactions in cardiac patients in India. A study conducted in South India demonstrated that hospitalized cardiac patients are at an increased risk of potential drug interactions (30.67%) [8].
Hence, the present study aims to analyze the potential DDI in patients of intensive cardiac care unit in terms of the mechanism and severity of such interactions.
Materials and Methods
This is a prospective observational study conducted in the intensive cardiac care unit, Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India for a period of six months from July 2015 to December, 2015. The study was undertaken after approval from the Institutional Ethics Committee and informed consent was obtained. A total of 500 patients of either sex were included in the study.
Patients aged 18 years and above, who admitted in the intensive cardiac care unit and those prescribed two or more drugs were included in the study. Patients with congenital heart disease and infectious cases were excluded.
Patient proforma was used for collecting the demographic details and medication profile of the patients. The pattern of potential DDI were analyzed using Medscape drug interaction checker [9]. Medscape contains a separate tool for detecting DDIs known as the multidrug interaction checker tool. When the drugs were entered in the tool, it displayed the potential DDIs and classified them based on severity as contra-indicated, serious (risk of life threatening drug interaction; use alternative drug), significant (potential for dangerous interaction, use with caution and monitor closely) and minor (non significant interaction) [10].
Statistical Analysis
The data was recorded in Microsoft excel 2010 worksheet. Descriptive statistics was used to summarize several demographic parameters and pDDI. All the statistical analyses were carried out using parametric tests with Statistical Package for Social Sciences (SPSS software version 16.0, IBM Corporation). A p-value<0.05 was considered as statistically significant with two tailed tests. One-way ANOVA followed by Bonferroni post-hoc analysis was performed to test the significance of severity and mechanism of DDI. A statistically significant association between variables was tested using Karl Pearson Coefficient of Correlation.
Results
A total of 500 patients were included in the study with the aim to identify pDDI among hospitalized cardiac patients in a tertiary care hospital.
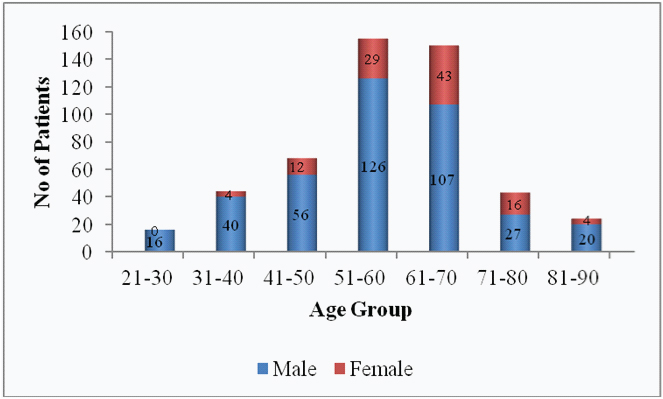
Demographic features: Majority of patients in the present study were in the age group of 51-60 years. The mean age was 55.5±12.6 years. Among 500 patients, 392 (78.4%) patients were males while 108 (21.6%) patients were females [Table/Fig-1]. The most common diagnosis in the study population was acute coronary syndrome (58.6%) followed by ischemic heart disease (20.4%) [Table/Fig-2].
Demographic pattern of study population.
Diagnosis of hospitalized cardiac patients in a tertiary care hospital.
| Diagnosis | Frequency(n= 500) | Percentage (%) |
|---|
| Acute coronary syndrome | 293 | 58.6 |
| Ischemic heart disease | 102 | 20.4 |
| Angina | 60 | 12 |
| Congestive heart failure | 4 | 0.8 |
| Others (hypertension, arrhythmias) | 41 | 8.2 |
Potential drug interactions: The total number of DDI was 2849 with a mean of 5.69±4.87 per patient. The most common interacting pairs of drugs were aspirin/clopidogrel (16.1%), pantoprazole/clopidogrel (9.8%), ramipril/aspirin (9.7%), aspirin/heparin (7.3%) and aspirin/metoprolol (7.1%).
Out of the total 2849 DDI, 2194 (77.01%) were pharmacodynamic while 586 (20.57%) were pharmacokinetic in nature. About 69 (2.42%) drug pairs interacted by unknown mechanism. Since, the difference among the three categories of mechanism of DDI (pharmacokinetic, pharmacodynamic and unknown) was significant, it was concluded that there is a difference in group means. Further, Bonferroni post-hoc analysis was performed and the p-value was obtained which confirmed that there is a statistically significant difference among the three categories. The post-hoc analysis assessed that which pairs of group means show statistically significant differences and which pairs do not show any such statistically significant differences as shown in [Table/Fig-3].
Bonferroni Post-hoc analysis for mechanism of DDI.
| (I) Group | (J) Group | Mean Difference(I-J) | Std. Error | p-value | Significance | 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| 1 | 2 | -3.21600 | .14989 | <0.001 | Extremely Significant | -3.5752 | -2.8568 |
| 3 | 1.03463 | .15027 | <0.001 | Extremely Significant | .6745 | 1.3948 |
| 2 | 1 | 3.21600 | .14989 | <0.001 | Extremely Significant | 2.8568 | 3.5752 |
| 3 | 4.25063 | .15027 | <0.001 | Extremely Significant | 3.8905 | 4.6108 |
| 3 | 1 | -1.03463 | .15027 | <0.001 | Extremely Significant | -1.3948 | -.6745 |
| 2 | -4.25063 | .15027 | <0.001 | Extremely Significant | -4.6108 | -3.8905 |
Groups: 1) Pharmacokinetic; 2) Pharmacodynamic; 3) Unknown
Majority of drug interactions were significant {2031 (71.29%)} in nature followed by minor {725 (25.45%)} while serious drug interactions were observed in only 93 (3.26%) drug pairs. Since the difference among the three categories of severity of DDI (minor, significant and serious) was significant, it was concluded that there is a difference in group means. Bonferroni post hoc analysis was then performed and the p-value obtained which confirmed statistically significant difference among the three groups [Table/Fig-4].
Bonferroni Post-Hoc Analysis for severity of DDI.
| (I) Group | (J) Group | Mean Difference(I-J) | Std. Error | p-value | Significance | 95% Confidence Interval |
|---|
| Lower Bound | Upper Bound |
|---|
| 1 | 2 | -1.51914 | .18022 | <0.001 | Extremely Significant | -1.9513 | -1.0869 |
| 3 | 2.32610 | .21530 | .089 | Not Significant | 1.8098 | 2.8424 |
| 2 | 1 | 1.51914 | .18022 | <0.001 | Extremely Significant | 1.0869 | 1.9513 |
| 3 | 3.84523 | .19242 | <0.001 | Extremely Significant | 3.3838 | 4.3067 |
| 3 | 1 | -2.32610 | .21530 | .089 | Not Significant | -2.8424 | -1.8098 |
| 2 | -3.84523 | .19242 | <0.001 | Extremely Significant | -4.3067 | -3.3838 |
Groups:1) Minor; 2) Significant; 3) Serious
The total number of DDI and their classification based on severity and mechanism has been summarized in [Table/Fig-5].
Number of drug-drug interactions and their classification.
| Variables | Total | Mean±SD |
|---|
| Number of drugs | 3591 | 7.27±3.17 |
| Total number of DDI | 2849 | 5.69±4.87 |
| Based on severity of DDI |
| Minor | 725 (25.45%) | 2.76±2.28 |
| Significant | 2031 (71.29%) | 4.28±2.84 |
| Serious | 93 (3.26%) | 0.43±0.50 |
| Based on mechanism of DDI |
| Pharmacodynamic | 2194 (77.01%) | 4.39±3.82 |
| Pharmacokinetic | 586 (20.57%) | 1.17±1.39 |
| Unknown | 69 (2.42%) | 0.14±0.49 |
The incidence of pDDI was correlated with different variables using Karl Pearson Correlation coefficient (r). There was a linear relationship between patient’s age and number of drugs prescribed (r = 0.178, p <0.001). A positive correlation was also observed between number of drugs prescribed and pDDI (r = 0.788, p<0.001) and between patient’s age and pDDI (r = 0.338, p<0.001).
Discussion
DDI is a major concern in the treatment of patients presenting with cardiovascular diseases as most of the cardiac patients present with comorbid conditions leading to prescription of multiple drugs. It has been observed that cardiac patients are more prone to drug interactions as compared to other patients [11]. The severity of DDI may vary from non significant interactions to serious or life threatening interactions. A study conducted by Askari M et al., has demonstrated that patients admitted in ICU had a number of clinically relevant DDI requiring intervention [12]. Identification of potential drug–drug interactions and assessment of their severity and mechanism among hospitalized cardiac patients is the need of the hour.
A number of different software programs are available which help to identify and assess the pattern of DDI. One of them which was used in the present study is the Medscape drug interaction checker [9]. Other available software programs include Micromedex Drug–Reax system. Mobile Micromedex Drug Information is a tool which allows the clinician to access the content of Micromedex through mobile phones. Lexi–Interact and WebMD interaction checker are the other database. Another one is Cerner Multum, Inc. which provides healthcare services in the United States. In India, the Maharashtra State Pharmacy Council (MSPC) has developed its own software to identify DDIs.
Most of the patients in this study were in the age group of 51-60 years (31%) which is similar to a previous study [10]. Males were considerably higher as compared to females which correlates with a study conducted in Western Nepal [13].
The most frequent drug interaction was observed with aspirin/clopidogrel combination. These drugs increase toxicity of each other by pharmacodynamic synergism leading to increased risk of bleeding tendencies. However, most of the cardiac patients are prescribed low dose aspirin and clopidogrel as the combination reduces the chances of subsequent cardiovascular events. Thus, the benefits outweigh the risks when used for the right indication and for the right duration [14]. The second most frequent drug interaction was observed at the level of metabolism with pantoprazole / clopidogrel combination which is identical to a study done earlier [15]. However, no dose adjustment of clopidogrel is required as mentioned in the Medscape interaction checker tool. The third most frequently encountered drug interaction was seen with ramipril/aspirin where aspirin may affect fluid homeostasis by decreasing the synthesis of renal prostaglandins and attenuate the antihypertensive effects of ramipril [16]. Our findings coincide with a study done earlier where the most frequent potential drug interactions involved blood coagulation modifiers [17]. Aspirin/heparin was the next most frequently interacting pairs of drugs. Simultaneous use of low dose aspirin and anticoagulant like heparin decreases the chances of ischemic events but the risk of bleeding tendencies has to be borne in mind [18]. Medscape interaction checker suggests close monitoring of patient as both increase toxicity of each other. Aspirin/metoprolol was the fifth most commonly encountered interacting pair of drugs. The interaction observed between the two drugs is pharmacodynamic antagonism as aspirin decreases prostaglandin synthesis. Moreover, both the drugs are known to increase serum potassium levels so close monitoring is required as mentioned in Medscape interaction checker. Antithrombotic agents and cardiovascular drugs are usually reported as the most common interacting drug groups in ICU settings [19,20].
Based on mechanism, DDI may be either pharmacodynamic or pharmacokinetic interactions. Pharmacodynamic interactions are observed when the two drugs modify the effects of each other directly [21]. A pharmacodynamic synergistic interaction may be desired by the prescriber if the two drugs potentiate the effects of each other as seen with low dose aspirin/clopidogrel in the present study. When a particular drug impedes the effects of another drug, it implies pharmacodynamic antagonism which was observed with ramipril/aspirin and aspirin/metoprolol combination in this study. On the other hand, pharmacokinetic interaction is seen when one drug alters the effects of the other drug at the level of absorption, distribution, metabolism or excretion of drug. In this study, DDI was seen at the level of metabolism with pantoprazole/clopidogrel combination.
Out of the total 2849 DDI, pharmacodynamic interactions were more frequently observed as compared to pharmacokinetic interactions. These findings are similar to a study conducted by Patel VK et al., [8]. Based on severity, most of the drug interactions were significant in nature followed by minor and serious drug interaction which is consistent with other study [16].
A statistically significant linear relationship was found between the number of drugs prescribed and pDDI (r = 0.788, p<0.001). These findings suggest that the potential for drug interaction increases with increase in the number of drugs as observed in similar other studies [22–24]. Similarly, a positive linear relationship was seen between patient’s age and number of drugs prescribed (r = 0.178, p<0.001) and between patient’s age and pDDIs (r = 0.338, p<0.001). Elderly patients usually present with co-morbid conditions and are on multiple drug therapy leading to increased risk of pDDIs. This result is consistent with the fact that the risk of pDDIs increases with age of the patient due to polypharmacy as seen in several other studies [25,26].
Limitation
The present study had some limitations of its own. Data was collected only from cardiac patients for a limited period. pDDI were analyzed using one of the many available software programs. Further studies should be undertaken in future involving more patients from various specialities and pDDI should be analyzed and compared using different available software programs to avoid any bias. Doctors and paramedical staff must be constantly sensitized about common DDI likely to be encountered in clinical practice so that it does not pose any risk to the patients.
Conclusion
The present study concludes that hospitalized cardiac patients are at an increased risk of pDDIs and provides the mechanism and severity of drug interactions among the most frequently prescribed drugs in cardiac patients. The risk of pDDIs was more commonly observed in elderly male patients particularly with antiplatelet drugs like low dose aspirin and clopidogrel. Understanding the mechanism and severity of pDDIs is of utmost importance to the prescriber to avoid the risks of adverse drug reactions and unnecessary financial burden to the patient.
Groups: 1) Pharmacokinetic; 2) Pharmacodynamic; 3) Unknown
Groups:1) Minor; 2) Significant; 3) Serious