Nepal is one of the developing countries in South Asia and the use of new technologies and innovations are out of access for most people living in this country [1]. The application of mechanical sewing machine is still in existence at large scale in most part of the Asian countries including Nepal; however, none of the epidemiological or related studies have been carried out to assess the prevalence of use of mechanical sewing machine and its impact on sural nerve. [2]. This is one of the major occupations adopted by skilled people with lower socioeconomic status and education level. Though, many skilled people adopted tailoring occupation and they are using paddle driven mechanical sewing machine [3]. Nerves of lower limbs including sural nerves are exposed to strenuous and chronic vibration exerted by chronic paddling and vibration effect of mechanical sewing machine [4]. Such type of continuous and chronic stress may cause structural, functional and pathological changes in them. Sural nerve is the pure sensory and peripheral nerve so it is much vulnerable to get affected by any type of stress or pathological condition [4]. Sural nerves are among the nerves commonly studied in the lower limbs and innervate the muscles of lateral and posterior third of leg as well as the lateral aspect of foot and heel. It is responsible for cutaneous sensation of the lower limbs [5].
The significance of sensory nerve conduction studies for diagnosing polyneuropathy is undisputed. On the other hand, in the diagnostic setting, such studies are usually performed only in upper limbs nerves despite the changes in polyneuropathy commonly present clinically first or more widely in the lower limbs, particularly in sural nerve [6]. Of all lower limb sensory nerves, only the sural provides such a ready index of lower limb sensory function. There can be little dispute that sensory nerve conduction studies in lower limb nerves have a higher percentage yield in polyneuropathy than such studies in upper limb nerves [7]. The study of sensory nerve conduction study of sural nerve does not need the complex techniques as required for the study of other peripheral sensory and motor nerves of upper and lower limbs. Sensory nerve conduction study of sural nerve has already been reported in healthy subjects and in the diagnosis of polyneuropathy [8].
The electro-diagnostic assessment includes two major components: Nerve Conduction Studies (NCS) and needle Electromyography (EMG) [9]. They are extension of neuro-physiological examination. Nerve conduction studies assess peripheral motor and sensory functions by recording the evoked response to stimulation of peripheral nerves by electrical stimulus. Sensory nerve conduction studies are performed by stimulating a mixed nerve or cutaneous nerve [10]. Nerve conduction study has been very useful for clinician in the diagnostic setting to investigate the extent and nature of neural lesions such as; demyelination and axonal degeneration [11].
There is a significant volume of evidence that the nervous system responds to increased physical activity by changes in its properties which is referred to as “neural adaptations to training” [12]. Early reports on changes in axon diameters in peripheral nerves have shown both increase [13,14] and decrease [15,16] with much variability in findings attributable to different intensities and types of exercise. A research conducted on rickshaw pullers has come up with the conclusion that muscle hypertrophy of lower limbs produced by hyperactivity has an influence on nerves supplying the muscles with encouraging changes in conduction velocities of sensory and motor nerves [17].
The guidelines for the electro-diagnostic tests, as the study of the nerve conduction, have been established for the general population and athletes but not for tailors or related others. The tailors using mechanical sewing machine are submitted to chronic strenuous paddling and vibration effect of mechanical sewing machine. There is need for study on the nerve conduction parameters in this particular population [18].
Though there are many reports on nerve conduction studies on athletes and trainees yet reports on tailor’s nerve conduction is still lacking [19]. It is rather discouraging that the tailoring occupation which employs great human potential has not been included in priority by researchers and stakeholders to study the chronic health hazards originated from vibration and strenuous paddling of mechanically operated sewing machine [2]. The chronic paddling and vibration of such sewing machine may produce some detrimental changes in nerves of lower limbs most probably sural nerve thereby affecting their nerve conduction parameters [4]. Therefore, this study, a novel and first of its kind, was destined to assess the sural nerves conduction parameters that might be altered due to chronic strenuous paddling and vibration effect of mechanical sewing machine.
We have conducted a larger study on the same population and this article form a part of our study. Till now, we have published an article entitled with a study to assess the Peak Expiratory Flow Rate (PEFR) in Nepalese population involved in tailoring occupation. Our study showed significantly reduced PEFR in study group in comparison to control group (p-value<0.05). This study indicates that tailors are more vulnerable to subclinical respiratory impairment due to chronic exposure to cloth dust in their working environment [20].
Materials and Methods
Subjects
The study was conducted in Neurophysiology Laboratory of Department of Basic and Clinical Physiology, BPKIHS, Dharan, Nepal for one year and two months (January 2011 to March 2012). The study population included 30 tailors (study group) and 30 healthy volunteers (control group). Average duration of occupation for study group was found to be 12.6 years. Inclusion criteria for control group as well as study group were those with ages 18 year to 60 year (a long range of age had been selected to get healthy subjects in both study group and control group. They were not clinically diagnosed with any systemic diseases (hypertension, diabetes etc.,), cardiovascular diseases, any neuropathic disease, neuromuscular diseases, musculoskeletal diseases, respiratory diseases, obesity and other chronic diseases on the basis of medical history and physical examination. They were not regular smokers and alcohol drinkers. They were not under any medication that is known to affect the nerve conduction study parameters. The study was approved by the ethical committee of BP Koirala Institute of Health Sciences as per its guidelines. Participants provided written informed consent. Among subjects those passed initial screening based on clinical history and physical examination were selected for Nerve Conduction Study (NCS).
Methods
Anthropometric and cardio-respiratory parameters (SBP, DBP, PR and RR) of the subjects were recorded. The nerves tested were the sural (both legs). The sites of stimulation and recording are shown in the [Table/Fig-1]. On each subject, antidromic sensory parameters of the nerves were measured. Antidromic for sensory nerve conduction study is when the recording is opposite to the physiological direction [21]. Digital Nihon Kohden Machine (NM-420S, H636, Japan) with its accessories was used for nerve conduction studies. The temperature of the recording room was kept around 26±20C.
Localizations of stimulating and recording electrodes used during sural nerve conduction study.
Source: Basic principles of nerve conduction study and electromyography. In: Misulis KE, Head TC editors. Essentials of clinical neurophysiology. Burlington; p. 127-160, 2003.
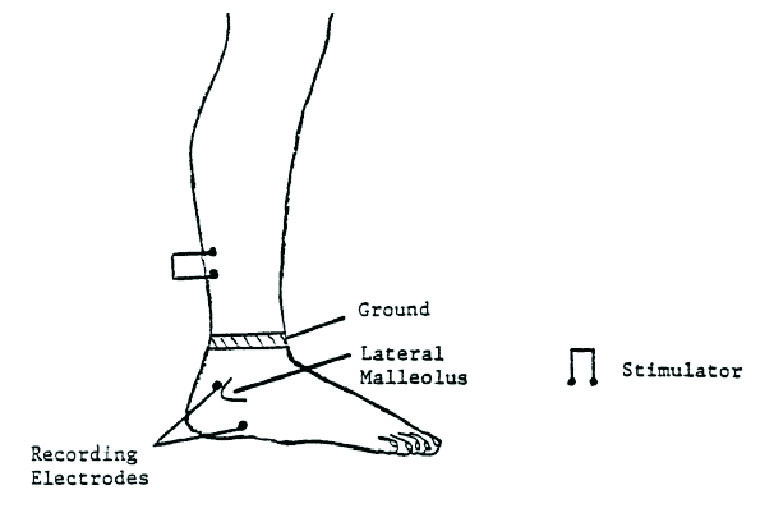
Sensory nerve conduction study Sural nerve conduction study
Antidromic method of stimulation was used for sural nerve. The active recording electrode was placed posterior to the lateral malleolus and the reference electrode 4 cm distal towards the lateral aspect of the foot [Table/Fig-1]. The ground electrode was placed just proximal to the active electrode.
The gain was set at 10-20 mV per division. An electrical pulse of 0.1 millisecond duration was used and the sural nerve was stimulated by stimulator with a current in the range of 16 to 30 mA to achieve supra maximal stimulation on the posterior portion of the calf 14 cm from the active electrode. The stimulating electrical stimulus was increased from a baseline of 0 mA, usually by 3-5 (mA) until the recorded sensory nerve action potential was maximized. Conduction velocity of nerve fibers, latency and amplitude of sural sensory nerve were recorded [Table/Fig-2] [22,23].
Representative trace of sensory (sural) nerve action potential of right and left legs.
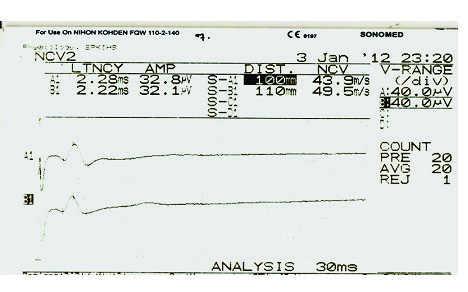
Statistical Analysis
The SPSS software (Statistical Package for Social Sciences, version-20.0) for personal computer was used for the statistical analyses. Kolmogorov-Smirnov test was applied to examine normality in the distribution of data. The variables those were found to be parametric were presented as Mean±SD and the remaining nonparametric variables were presented as Median (Q1-Q3). The differences in variables between the study and control groups were tested using Student’s t-test for parametric variables and Mann-Whitney U test for nonparametric variables. A p-value of < 0.05 was considered significant.
Results
We did not find any significant difference among participants of study group on the basis of duration of their tailoring profession. Both, the study group, and control group formed a very homogenous population without significant differences in age, height, length of right and left legs, weight, and body mass index [Table/Fig-3]. Resting heart rate, systolic blood pressure, diastolic blood pressure and respiration rate were also not significantly different between tailors and controls [Table/Fig-4].
Comparison of anthropometric variables between study group (tailors) and control group (controls).
| Variables | Tailors (n=30) | Controls (n=30) | p-value |
|---|
| *Age (years) | 34(31-37.25) | 34(32-36.25) | 0.651 |
| *Wt (kg) | 64.5(60-67) | 65(62-68) | 0.302 |
| Ht (m) | 1.64±0.05 | 1.65±0.05 | 0.227 |
| LORL (cm) | 84.14±3.39 | 83.78±5.09 | 0.699 |
| LOLL (cm) | 84.14±3.39 | 83.78±5.09 | 0.699 |
| BMI (kg/m2) | 23.93±1.8 | 23.95±1.44 | 0.955 |
p-value ≤ 0.05 = significant.
Wt: Weight, Ht: Height, BMI: Body mass index, LORL: Length of right leg, LOLL: Length of left leg.
Comparison of cardio-respiratory variables between study group and control group.
| Variables | Tailors (n=30) | Controls (n=30) | p-value |
|---|
| *SBP (mmHg) | 122(118-123.5) | 118(117.5-122) | 0.099 |
| *DBP (mmHg) | 78(76-82) | 80(78-82) | 0.513 |
| *PR (beat/min) | 71(69-72) | 71(70-72) | 0.087 |
| *RR (cycle/min) | 16(14.75-18) | 16(15.75-17.25) | 0.567 |
p-value ≤ 0.05 = Significant SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PR: Pulse rate, RR: Respiration rate.
The distance between stimulating and recording sites (Dist.SRS) of both right and left lower limbs was not significantly different between tailors and controls [Table/Fig-5,6].
Comparison of right sural sensory nerve conduction study variables between study group and control group.
| Variables | Tailors (n=30) | Controls (n=30) | p-value |
|---|
| *L-SNAP (ms) | 2.6(2.2-2.7) | 2.0(2.0-2.25) | 0.024 |
| Amp-SNAP (μv) | 20.75±5.42 | 24.10±5.45 | 0.020 |
| *Dist.SRE (cm) | 110(110-120) | 110(107.5-112.5) | 0.511 |
| *SNCV (m/s) | 44.23(42.72-47.83) | 50(46-54) | 0.001 |
p-value ≤ 0.05 = significant L-SNAP: Latency- Sensory nerve action potential, Amp-SNAP: Amplitude- Sensory nerve action potential, Dist.SRS: Distance between stimulating and recording electrode, SNCV: Sensory nerve action potential.
Comparison of left sural sensory nerve conduction study variables between study group and control group.
| Variables | Tailors (n=30) | Controls (n=30) | p-value |
|---|
| Value | Value |
|---|
| *L-SNAP(ms) | 2.4(2.07-2.72) | 2.0(2.0-2.0) | 0.020 |
| *Amp-SNAP(μv)) | 18.2(12.43-21.8) | 32.0(26.5-35.25) | 0.001 |
| *Dist.SRE(cm) | 110(107.5-110) | 110(100-110) | 0.373 |
| SNCV(m/s) | 45.967±5.86 | 50.67±6.59 | 0.005 |
The p-value ≤ 0.05 = Significant
Sensory nerve conduction velocities and amplitudes of both the right and left sural nerves were significantly lower in tailors in comparison to controls as shown in [Table/Fig-5,6].
Latencies of both the right and left sural nerves were significantly more in tailors as compare to controls as shown in [Table/Fig-5,6].
The differences in variables between the subject and control groups were tested using Student’s t-test for parametric variables and Mann-Whitney U test for nonparametric variables.
Note: variables with (*) indicates Mann-Whitney U test for nonparametric variables and without asterisk indicates t-test for parametric variables.
The differences in variables between the subject and control groups were tested using Student’s t-test for parametric variables and Mann-Whitney U test for nonparametric variables.
Note: variables with (*) indicates Mann-Whitney U test for nonparametric variables and without asterisk indicates t-test for parametric variables.
Right sural sensory nerve
The differences in variables between the subject and control groups were tested using Student’s t-test for parametric variables and Mann-Whitney U test for nonparametric variables.
Note: variables with (*) indicates Mann-Whitney U test for non-parametric variables and without asterisk indicates t-test for parametric variables.
Left sural sensory nerve
The differences in variables between the subject and control groups were tested using Student’s t-test for parametric variables and Mann-Whitney U test for nonparametric variables.
Note: variables with (*) indicates Mann-Whitney U test for nonparametric variables and without asterisk indicates t-test for parametric variables.
Discussion
In the present study, we evaluated the sensory nerve conduction parameters of both right and left sural nerves of tailors (study group) and normal healthy volunteers (control group). Both, right and left sural SNAP onset latencies, were found significantly prolonged (p≤0.05) in tailors, whereas, their amplitudes and conduction velocities were found to be significantly low (p≤0.05) in them as compared to those of controls. Our results did not show any significant difference of age variation, so the age factor could not have caused any net effect on the nerve conduction study parameters of sural nerves.
SNAP latency: In our study, the sensory onset latencies of both the lower limbs were significantly prolonged. Our study came out with the similar result to a study by Bovenji M et al., [24]. They showed that there were significantly prolonged latencies of sensory nerves in workers of vibrating tool industry. Prolongation of latencies in our study could be attributable to either reduced number of fast conditioning sensory fibers or substantial reduction in excitability of the same nerves. However, certain contrasting findings are also available. A study by Gordon T et al., showed the reduced latencies of both right and left sural nerves in marathoner and they concluded that there was activity induced morphologic changes in sural nerves [16]. The study of Nobue A et al., also showed that there were improved latencies of sural nerves in sprint runners [13]. So, our results are in contrast with the studies of Gordon T et al., and Nobue A et al. [13,16].
SNAP amplitude: Study of Feinberg JH et al. revealed significant reduction in amplitude of sural nerves of both, the right and left legs, of hockey players suffering from chronic numbness in legs [25]. Bovenji M et al., also found significantly reduced amplitude of sural nerves of both the limbs in vibration tool industry workers [24]. The study conducted by Colack T et al., showed that there was slight increase in the amplitude of the sural nerves of long distance runners [26]. Our results are in the same direction as in the study by Feinberg JH et al., and Bovenji M et al. However, this study is in contrast with the study by Colack T et al., [24,26]. Findings could outline trend towards either axonal loss or conduction block in the axon of sural nerves.
SNAP conduction velocity: Ross A et al., found that there was increase in nerve conduction velocity of sural nerves bilaterally in the population of long distance runners [27]. Hirata M et al., found that there was poorer nerve conduction velocity of tibial, common peroneal and sural nerves of lower limbs in vibration syndrome and reduced muscle mass as well [28]. Our study has similar findings as in the study by Hirata M et al., However, it is in contrast with the study of Ross A et al. Reduced nerve conduction velocity of sural nerves in both legs could be attributable to either axonal loss of fast conducting nerve fibers or demyelination.
Poor SNCS in tailors as compared to controls might be due to vibration effect of mechanical sewing machine on sural nerve conduction parameters. The effect of this type of exercise (regular and chronic paddling) has not been able to counter the detrimental effect of vibration on sural nerves of both the legs [24]. Vibration of mechanical sewing machine could have masked the effect of chronic paddling of lower limbs in tailors. Further, Untunen et al., have suggested that there may be polyneuropathy and muscle weakness in operators with vibration syndrome who had been working on vibrating tools for a long time [29].
Limitation
Findings of our study would have been more conclusive if the sample size had been larger and if all the peripheral nerves of lower limbs had been included in this study. Further, it could have been confirmative of either axonal loss or demyelination if histology of all the peripheral nerves of both legs had been studied microscopically.
Conclusion
Our results revealed that the reduction in amplitude and conduction velocity of SNAP of sural nerves of both legs in tailors whereas, prolongation of onset latency of SNAP of corresponding nerves in the same group as compared to control group could be due to vibration effect of mechanical sewing machine on sural nerves. Vibration effect of mechanical sewing machine possibly overshadowed the exercising effect of the mechanical sewing machine on lower limbs in tailors thereby suggesting trends towards subclinical neuropathy of sural nerves of both legs. Information obtained from the study will help to further assess the degree of assault on the sural nerves of both the right and left legs and recommend appropriate preventive measures that would ensure a healthier workforce in this type of occupation.
p-value ≤ 0.05 = significant.
Wt: Weight, Ht: Height, BMI: Body mass index, LORL: Length of right leg, LOLL: Length of left leg.
p-value ≤ 0.05 = Significant SBP: Systolic blood pressure, DBP: Diastolic blood pressure, PR: Pulse rate, RR: Respiration rate.
p-value ≤ 0.05 = significant L-SNAP: Latency- Sensory nerve action potential, Amp-SNAP: Amplitude- Sensory nerve action potential, Dist.SRS: Distance between stimulating and recording electrode, SNCV: Sensory nerve action potential.
The p-value ≤ 0.05 = Significant