Molecular Characterization of Multidrug Resistant Strains of Acinetobacter baumannii Isolated from Intensive Care Units in West of Iran
Parviz Mohajeri1, Abbas Farahani2, Rasa Sheini Mehrabzadeh3
1 Associate Professor, Department of Microbiology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2 Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; PhD Candidate, Research Assistant, Department of Microbiology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3 PhD Student, Research Assistant, Department of Pathobiology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rasa Sheini Mehrabzadeh, Department of Pathobiology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
E-mail: rasa.mehrabzade@gmail.com
Introduction
According to the results of various studies using phenotypic methods, the prevalence of Multidrug Resistant (MDR) Acinetobacter baumannii (A. baumannii) isolates has been increasing worldwide. Pulsed-Field Gel Electrophoresis (PFGE) technique is known as the gold standard method to determine clonal characterization of bacterial species, especially A. baumannii.
Aim
To determine the clonal relatedness and investigate the prevalence of integron classes 1 and 2 and genes encoding OXA-23 and 24 in A.baumanii isolates.
Materials and Methods
A cross-sectional study was conducted from November 2011 to January 2013. A total of 140 A.baumannii isolates collected from three hospitals of Kermanshah were considered out of which 75 ICU isolates were included in this study. Antibiotics susceptibility test was done by disk diffusion method. Polymerase Chain Reaction (PCR) was performed in order to detect class 1 and 2 integrons and blaOXA-23-like, blaOXA-24-like genes. Isolates identified as MDR from a total of 75 Intensive Care Units (ICU) strains were subjected to genotyping for clonal relatedness.
Results
A total of 37 isolates among 75 ICU isolates were identified as MDR. The maximum drug resistance was observed against ceftriaxone, mezlocycline, cefotaxime, piperacilin, ciprofloxacin and imipenem. Frequency of Class 1 and Class 2 Integrons, blaOXA-23-like and blaOXA-24-like genes were 33(44%), 27(36%), 60(80%) and 14(18.6%) respectively. Four clusters with high level of similarity were obtained showing homogeneity among MDR isolates.
Conclusion
Significant correlation between presence of integrons and resistance to different classes of antibiotic was observed in this study. Monitoring of drug resistance using gene integrase PCR and blaOXA gene by cluster analysis is very important to plan specific infection control measures due to MDR A. baumannii.
Ceftriaxone, Genotyping, Integron, OXA gene
Introduction
A.baumannii has emerged as an important nosocomial pathogen in the healthcare setting. Acinetobacter has become a risk factor of infections in hospitals particularly Carbapenem Resistant A.baumannii (CRAB) which is an increasing problem in this area in recent years [1].
The carbapenem class has been considered as a choice for treating serious A.baumannii infections for many years [2]. According to the results of various studies using phenotypic methods, the prevalence of MDR A.baumannii isolates has been increasing worldwide and has become one of the most difficult pathogen to treat [2,3]. The frequency of MDR A.baumannii isolates increased between 2001- 2007 compared to 2010 to 2013 in Iran. [2,3] Resistance to carbapenem has shown a dramatic increase between 2010 and 2013 [3]. According to studies done by PCR-based molecular methods, resistant genes has greatly spread from 2001 to 2013 which indicates a quick gene transfer between isolates through mobile genetic elements or resistance transfer factor plasmids [3,4].
Mobile genetic elements including integrons, plasmids and transposons are the main resistance transfer factors in Gram-negative bacteria especially A.baumannii. A number of studies have shown that, there is a direct relationship between drug resistant isolates and the existence of chromosomal mobile elements [4,5].
The rate of A.baumannii resistance to imipenem is considerably lower than the rates found in the neighboring countries including Saudi Arabia (63%), United Arab Emirates (76%), Turkey (98%) and Pakistan (100%) compared to Iran [6]. Typing methods play an important role in assessing interspecies communication. Spread studies can be done easily using these methods and species diversity can be identified [2,7]. Now-a-days, different typing methods such as PCR based methods {Enterobacterial Repetitive Intergenic Consensus (ERIC) and Random Amplified Polymorphic DNA (RAPD)}, Variable Number Tandem Repeat (VNTR), PFGE typing has been proposed to determine the common clones of A.baumannii [7,8].
PFGE technique is known as the gold standard method used to identify bacterial species, especially A.baumannii [2]. Although, it is arduous, required equipments are already available not only at Reference laboratories, but also at some of the advanced laboratories of the hospitals [9]. Knowledge of clonal outbreaks is of importance in epidemiological studies for A.baumannii infections [2]. Thus, the present study was conducted to determine clonal relatedness between MDR strains of A.baumanii isolates.
Materials and Methods
A cross-sectional study was conducted from November 2011 to January 2013. A total of 140 A.baumannii isolates from clinical samples (blood, sputum, wounds, urine, abdominal abscesses, synovial) identified using biochemical tests and kit API 20 NE, obtained from three hospitals of Kermanshah region, Iran was considered and of these only 75 A.baumannii ICU strains were included in this study. Isolates identified as MDR from these 75 ICU strains were subjected to genotyping (PFGE). The MDR determination was done according to the criteria as described previously [2]. Isolates resistant to at least three classes of anti-microbial agents (all penicillins and fluoroquinolones, cephalosporins, and aminoglycosides) were identified as MDR.
Kirby Bauer method was done according to the CLSI guidelines [10] for 20 antibiotics: levofloxacine (5 μg), gatifloxacin (5 μg), ciprofloxacin (5 μg), tobramycin (10 μg), gentamycin (10 μg), tigecycline (15 μg), amikacin (30 μg), meropenem (10 μg), imipenem (10 μg), piperacilin (100 μg), mezlocycline (75 μg), cotrimoxazole (30 μg), polymixine b (300 unit), colistin (10 μg), tetracycline (30 μg), minocycline (30 μg), cefepime (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg).
DNA extraction and PCR: Bacterial DNA was extracted from A. baumannii isolates by boiling. PCR was carried out for amplification of genes in MDR isolates. Primers for Classes 1 and 2 Integron genes were designed as previously described by Mirnejad et al., [5] and blaOXA-23-like and blaOXA-24-like genes previously described by other authors [2].
Pulsed-field gel electrophoresis analysis and dendrogram construction: MDR strains were subjected to PFGE analysis using the methods as described by Mohajeri et al., [2]. The DNA banding patterns were analysed using Bionumeric 7.0 software (Appllied maths NV, St-Martens-Latem Beligum). Cut off levels of 85 and 100% were applied to this dendrogram.
Results
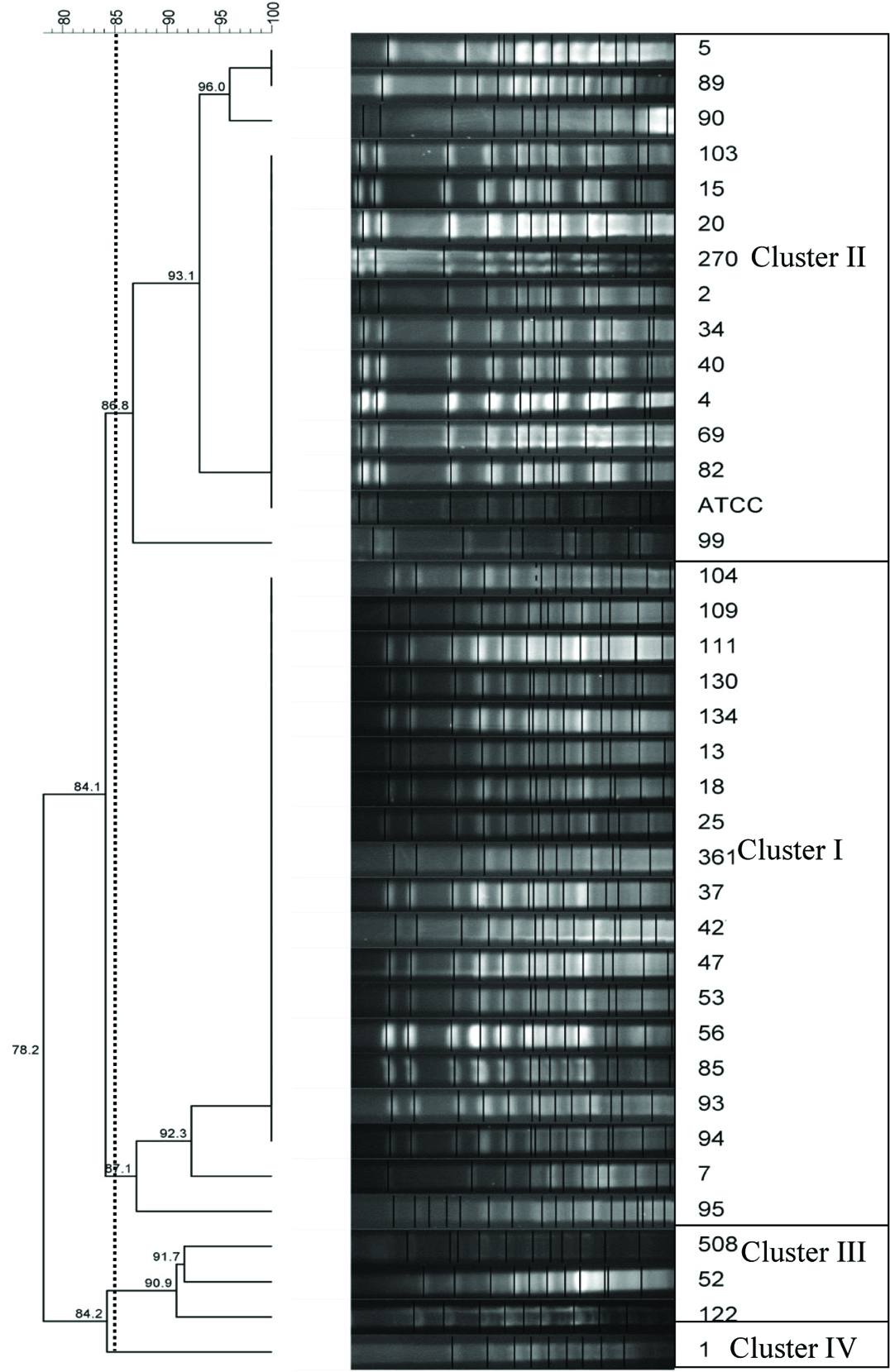
Thirty seven isolates were MDR among 75 isolates. High resistance was observed to mezlocillin, ceftriaxone, cefotaxime (>90%) and imipenem, ceftazidime, levofloxacine, piperacillin (>80%) [Table/Fig-1] and susceptible to colistin, polymyxin B and tetracycline. Frequency of Class 1 and Class 2 Integrons, blaOXA-23-like and blaOXA-24-like were 33(44%), 27(36%), 60(80%) and 14(18.6%) respectively. Resistance to cephalosporins, carbapenemes, fluoroquinolones, penicillin and tetracycline were related to coexist in integrons and blaOXA genes [Table/Fig-1]. Significant correlation between presence of integron’s and resistance to different of antibiotic classes was observed. A total of 13 (35.1%), 10 (27%) pulsotypes were positive for integron Classes1, 2, and 32 (86.5%), 9 (24.3%) pulsotypes were positive for blaOXA-23-like and blaOXA-24-like in MDR isolates, respectively [Table/Fig-2]. Four clusters were obtained through PFGE, cluster I and II were the most frequent isolates with 14 and 19 isolates and were present to the greatest extent, after them, there were cluster number three and four with three and one isolates, which were considered as a single clone [Table/Fig-3]. High homogeneity was observed among MDR isolates.
Distribution of resistance in 75 clinical A.baumannii isolates with arrangement by antibiotic Classes, Integrons and OXA genes.
| Antibiotic Class | Tested Members | Antibiotic Susceptibility | Integron-Positive1 in MDR Isolates | OXA- Positive2 in MDR Isolates |
|---|
| S | I | R | S | I | R | S | I | R |
|---|
| Fluorqinolones | Levofloxacine | 17 | 5 | 53 | 9 | 2 | 12 | 12 | 3 | 26 |
| Gatifloxacin | 26 | 8 | 41 | 11 | 2 | 10 | 14 | 5 | 20 |
| Ciprofloxacin | 10 | 0 | 65 | 3 | 0 | 20 | 8 | 0 | 33 |
| Aminoglycoside | Tobramycin | 34 | 6 | 35 | 10 | 2 | 11 | 21 | 3 | 17 |
| Gentamicine | 14 | 0 | 61 | 8 | 0 | 15 | 14 | 0 | 27 |
| Tigecycline | 73 | 1 | 1 | 19 | 2 | 2 | 38 | 1 | 1 |
| Amikacin | 19 | 9 | 47 | 12 | 3 | 8 | 17 | 4 | 20 |
| Carbapenem | Meropenem | 13 | 5 | 57 | 3 | 4 | 16 | 1 | 3 | 36 |
| Imipenem | 10 | 3 | 62 | 4 | 1 | 18 | 2 | 2 | 37 |
| Spread range of penicillin | Piperacilin | 9 | 0 | 66 | 7 | 0 | 16 | 11 | 0 | 30 |
| Mezlocycline | 3 | 0 | 72 | 0 | 0 | 23 | 0 | 2 | 39 |
| Sulfonamide | Cotrimoxazole | 24 | 1 | 50 | 11 | 0 | 12 | 19 | 0 | 22 |
| Polymixin B | 65 | 0 | 10 | 21 | 0 | 2 | 35 | 0 | 6 |
| Colistin | 67 | 0 | 8 | 21 | 0 | 2 | 37 | 0 | 4 |
| Tetracycline | Tetracycline | 13 | 1 | 61 | 3 | 0 | 20 | 12 | 0 | 29 |
| Minocycline | 54 | 6 | 15 | 13 | 1 | 9 | 27 | 1 | 10 |
| Cephalosporins | Cefepime | 11 | 0 | 64 | 4 | 0 | 19 | 9 | 0 | 32 |
| Cefotaxime | 4 | 0 | 71 | 0 | 0 | 23 | 0 | 0 | 41 |
| Ceftazidime | 11 | 0 | 64 | 5 | 0 | 18 | 9 | 0 | 32 |
| Ceftriaxone | 3 | 0 | 72 | 0 | 1 | 22 | 0 | 3 | 37 |
1Integron-positive isolates= Integron I + Integron II
2OXA- Positive= blaOXA-23-like + blaOXA-24-like
S: Susceptible
I: Intermediate
R: Resistant
Distribution of pulsotypes, classes 1 and 2 integron, blaOXA-23-like and blaOXA-24-like and resistance phenotypes among A.baumannii isolates.
| Pulsotypes | Integron 1 | Integron 2 | OXA-23 | OXA-24 | MBL1 |
|---|
| N | P | N | P | N | P | N | P | N | P |
|---|
| Cluster I | 13 | 6 | 11 | 8 | 3 | 16 | 14 | 5 | 4 | 15 |
| Cluster II | 8 | 6 | 12 | 2 | 2 | 12 | 12 | 2 | 2 | 12 |
| Cluster III | 2 | 1 | 3 | 0 | 0 | 3 | 1 | 2 | 0 | 3 |
| Cluster IV | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Total | 37 | 37 | 37 | 37 | 37 |
N= Negative, P= Positive
1MBL= metalo-β-lactamase
PFGE profiles of ApaI-digested genomic DNA for clinical A.baumannii isolates.
Discussion
Antibiotic resistant genes and integrons in A.baumannii strains have emerged as a main problem in treatment of infections caused by these bacteria in recent times [11]. Infection by A.baumannii causes high incidence of morbidity and mortality among hospitalized patients [12]. In the present study, similar to Mirnejad et al., study, there was a significant correlation between presence of integrons and resistance to cephalosporins, ofloxacin, norfloxacin [5]. Class 1 and 2 integron are the most common among the Acinetobacter isolates and other clinical Gram negative bacteria [13–15]. A total of 63.5% of A.baumannii isolates contained class1 Integrons in Koczura’s and his colleagues’ study [16] conducted in 2014, Poland and there were no class 2 integrons in isolates while both of these integrons were present in our study. A total of 53% of Acinetobacter isolates contained Class 1 or 2 or both in a study of Japoni et al., conducted at 2011 in Shiraz and their results were the same as ours [17]. The number of Class 1 integrons was more than Class 2 in a study of Moammadi F et al., which lines with our study [18]. Frequency of Class 1 Integrons (92.5%) was reported more than Class 2 and MDR isolated were the most frequent isolates in a study of Peymani et al., [11]. In our study, 44% of isolates contained Class 1 integrons and 36% contained Class 2. In contrast with Peymani’s study, MDR isolates Class 1 Integrons were less frequent (35%). In contrast to our study, 42% of A.baumannii isolates contained Class 1 Integrons and 82% contained Class 2 in a study [5]. More than 50% of penicillins and cephalosporins resistant isolates contained Integrons in another study [15]. Ceftriaxone and cefotaxime resistant isolates had the highest frequency of Class 1 Integrons. This is perhaps surprising since, resistance to antibiotic compounds is often resulted from point mutations by chromosomal element such as Insertion Sequence (IS) element. Tetracycline resistant isolates also consists of most number of Class 2 Integrons which were same as our results [Table/Fig-1]. PFGE is known as the gold standard genotypic technique to investigate the molecular epidemiology of bacteria especially for nosocomial infection outbreaks [2]. The frequency of blaOXA-23-like genes was reported 93% among MDR isolates in the previous study of Mohajeri et al., same as this study [2]. Isolates analysed by PFGE had more diversity in contrast with the present study [2]. blaOXA-23-like gene was introduced as class D Carbapenem’s coding main factor similar to our results [19] conducted in 2007. blaOXA-23-like gene was also dominant among isolates of United States of America same as our study. Although, there were no blaOXA-24-like genes found in their study [20]. In this study, MDR A. baumannii clinical isolates showed lots of similarity (Between, 86.8-100%) suggesting the involvement of similar subtypes of the species in ICU infection. As previously reported, the ability of PFGE assay to determine main types and clusters association with infection can be used in outbreaks by nosocomial pathogens such as A. baumannii. Prevalence of blaOXA-23-like and integrons Classes 1 and 2 among MDR strains of A. baumannii are high in Kermanshah as indicated in this study.
Limitation
One of the major limitations of this study was sample size and inadequate demographic information and underlying disease.
Conclusion
Monitoring of drug resistance with use of coexist gene integrase PCR and blaOXA gene with cluster analysis are very important to plan specific infection control measures due to MDR A.baumannii in hospital settings. By PFGE analysis, we determined two main highly similar clusters (I and II) which stated genetic correlation between them.
Responsible patterns of infection prevalence in a hospital have genetic relationship and may have the same genetic source. Designing protection programs has a great importance such as existing infection control in different parts of the hospitals especially in the ICU. Nevertheless, further studies need to be carried out to characterize other genetic elements with integrons in these isolates and determine clonal lineage with Multilocus Sequence Typing (MLST) methods.
1Integron-positive isolates= Integron I + Integron II
2OXA- Positive= blaOXA-23-like + blaOXA-24-like
S: Susceptible
I: Intermediate
R: Resistant
N= Negative, P= Positive
1MBL= metalo-β-lactamase
[1]. Mohajeri P, Sharbati S, Farahani A, Rezaei Z, Evaluate the frequency distribution of non adhesive virulence factors in carbapenemase producing Acinetobacter baumannii isolated from clinical samples in KermanshahJ Nat Sci Biol Med 2016 7(1):58-61. [Google Scholar]
[2]. Mohajeri P, Farahani A, Feizabadi MM, Norozi B, Clonal evolution multidrug resistant Acinetobacter baumannii by pulsed-field gel electrophoresisIndian J Med Microbiol 2015 33(1):87-91. [Google Scholar]
[3]. Moradi J, Hashemi FB, Bahador A, Antibiotic resistance of Acinetobacter baumannii in Iran: A systemic review of the published literatureOsong Public Health Res Perspect 2015 6(2):79-86. [Google Scholar]
[4]. Cambray G, Guerout AM, Mazel D, IntegronsAnnu Rev Genet 2010 44:141-66. [Google Scholar]
[5]. Mirnejad R, Mostofi S, Masjedian F, Antibiotic resistance and carriage Class 1 and 2 integrons in clinical isolates of Acinetobacter baumannii from Tehran, IranAsian Pac J Trop Biomed 2013 3(2):140-45. [Google Scholar]
[6]. Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter sppInt J Antimicrob Agents 2006 27:351-53. [Google Scholar]
[7]. Rafei R, Pailhories H, Hamze M, Eveillard M, Mallat H, Dabboussi F, Molecular epidemiology of Acinetobacter baumannii in different hospitals in Tripoli, Lebanon using bla OXA-51-like sequence-based typingBMC Microbiol 2015 15:103 [Google Scholar]
[8]. Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, RodriguezValera F, Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumanniiJ Clin Microbiol 2005 43:4382e90 [Google Scholar]
[9]. Khosravi AD, Mehrabzadeh RS, Farahani A, Jamali H, Molecular identification of clinical isolates of mycobacterium fortuitum by Random Amplified Polymorphic DNA (RAPD) polymerase chain reaction and ERIC PCRJ Clin Diagn Res 2015 9(12):DC01-5. [Google Scholar]
[10]. Clinical and Laboratory Standards Institute, Performance standards for antimicrobial susceptibility testing; Twentieth informational supplement, CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA, 2014 [Google Scholar]
[11]. Peymani A, Farajnia S, Nahaei MR, Sohrabi N, Abbasi L, Ansarin K, Prevalence of Class 1 integron among multidrug resistant Acinetobacter baumannii in Tabriz, northwest of IranPol J Microbiol 2012 61(1):57-60. [Google Scholar]
[12]. Mohajeri P, Farahani A, Feizabadi MM, Ketabi H, Abiri R, Najafi F, Antimicrobial susceptibility profiling and genomic diversity of Acinetobacter baumannii isolates: A study in western IranIran J Microbiol 2013 5(3):195-202. [Google Scholar]
[13]. Lavakhamseh H, Mohajeri P, Rouhi S, Shakib P, Ramazanzadeh R, Rasani A, Multidrug resistant Escherichia coli strains isolated from patients are associated with Class 1 and 2 IntegronsChemotherapy 2016 61(2):72-76. [Google Scholar]
[14]. Martinez-Freijo P, Fluit AC, Schmitz FJ, Grek VS, Verhoef J, Jones ME, Class I integrons in Gramnegative isolates from different european hospitals and association with decreased susceptibility to multiple antibiotic compoundsJournal of Antimicrobial Chemotherapy 1998 42(1):689-96. [Google Scholar]
[15]. Taherikalani M, Maleki A, Sadeghifard N, Mohammadzadeh D, Soroush S, Asadollahi P, Dissemination of Class 1, 2 and 3 integrons among different multidrug resistant isolates of Acinetobacter baumannii in Tehran hospitals, IranPol J Microbiol 2011 60(2):169-74. [Google Scholar]
[16]. Koczura R, Przyszlakowska B, Mokracka J, Kaznowski A, Class 1 integrons and antibiotic resistance of clinical Acinetobacter calcoaceticus-baumannii complex in Poznan, PolandCurr Microbiol 2014 69(3):258-62. [Google Scholar]
[17]. Japoni S, Japoni A, Farshad S, Ali AA, Jamalidoust M, Association between existence of integrons and multi-drug resistance in Acinetobacter isolated from patients in southern IranPol J Microbiol 2011 60(2):163-68. [Google Scholar]
[18]. Moammadi F, Arabestani M, Safari M, Roshanaii G, Alikhani M, Prevalence of Class 1, 2 and 3 integrons among extensive drug resistance Acinetobacter baumanii strains isolated from intensive care units in Hamadan, west province, IranIran J Med Microbiol 2014 8(3):8-14. [Google Scholar]
[19]. Mendes RE, Bell JM, Turnidge JD, Castanheira M, Jones RN, Emergence and widespread dissemination of OXA-23, -24/40 and -58 carbapenemases among Acinetobacter spp. in Asia-Pacific nations: report from the SENTRY Surveillance ProgramJ Antimicrob Chemother 2009 55:59-63. [Google Scholar]
[20]. Srinivasan VB, Rajamohan G, Pancholi P, Stevenson K, Tadesse D, Patchanee P, Genetic relatedness and molecular characterization of multidrug resistant Acinetobacter baumannii isolated in central Ohio, USAAnn Clin Microbiol Antimicrob 2009 8:8-10. [Google Scholar]