Hypertension is the most common medical problem encountered during pregnancy. PIH is defined as a direct result of the gravid state. American Congress of Obstetricians and Gynaecologists (ACOG) classified hypertensive disorders during pregnancy as chronic hypertension preceding pregnancy; gestational hypertension; pregnancy-induced hypertension- pre-eclampsia, mild, severe; eclampsia; chronic hypertension with superimposed PIH- superimposed pre-eclampsia and superimposed eclampsia [1].
The relatively new theory of endothelial injury explains many of the clinical findings in pre-eclampsia. Primarily, there is placental dysfunction leading to a syndrome of endothelial dysfunction with associated vasospasm [8]. It was hypothesised that intermittent placental perfusion, secondary to deficient trophoblast invasion of the endometrial arteries, leads to an ischemia–reperfusion-type insult and results in the generation of free radicals [9,10]. Free radicals attack fatty acids in cell membranes and lipid hydroperoxides are formed. Consequently, lipid peroxides may cause endothelial dysfunction and an increase in sensitivity to vasopressors in pre-eclampsia [11–13].
It is envisaged that increased free radical activity arises from increased production of free radicals or deficiency in protective antioxidant system. The aim of the present study was to study the role of reactive nitrogen species in PIH.
Materials and Methods
Institutional ethical approval was obtained before the commencement of study. The place of study was Department of Biochemistry, Dr. V. M. Government Medical College, Solapur, Maharashtra, India. The study duration was from October 2010 to October 2012. Informed written consents were taken from patients. Sixty patients presenting with PIH in obstetrics and gynaecology department without any complaint of urinary tract infection, bronchopneumonia, hepatitis, influenza or any infection served as cases. Sixty pregnant females without PIH and any infection served as control. Oxidative stress plays a dual role in infections. Free radicals protect against invading organisms and they can also cause tissue damage during the resulting inflammation. In the process of infection, there is generation of reactive species by nitric-oxide synthase. So, reactive nitrogen species are abundant in infectious conditions. Hence, all infectious conditions like urinary tract infection, bronchopneumonia, hepatitis, influenza etc., were excluded from this study.
Collection of Samples
The blood samples were collected from the Department of Gynaecology. Total 5 ml sample was collected in plain red top tube containing no anticoagulant; serum was separated and used for the study. The estimation of serum nitric oxide, serum nitrothiol and serum total thiol was done by Griess method, Cook method and Habeeb method respectively.
Estimation of Serum Nitric Oxide by Griess Method [
14]
Principle: Nitric-oxide is measured in terms of nitrite and nitrate. It involves formation of chromophore during the reaction of nitrite (NO2-) with sulphanilamide and N (1-naphthyl) ethylenediamine (Griess reagent) forming pink coloured compound with a characteristic absorption spectra and λ max at 545 nm. Nitrate (NO3-) does not undergo diazotization reaction with sulphanilamide. Hence, it is first reduced quantitatively to nitrite by nitrate reductase enzyme. Nitrite reacts with Griess reagent. The colour obtained is due to reaction of nitrite already present in the sample and nitrite formed by reduction of nitrate. Thus, both nitrate and nitrite i.e., nitric-oxide are measured.
Method:E. coli containing nitrate reductase was prepared using E. coli bacteria. The pelleted bacteria were suspended and washed by centrifugation in cold Phosphate Buffer Saline (PBS) of pH 7.4. The washing of bacteria was repeated 10 times to completely remove nitrate. Every time the bacteria were pelleted by centrifuge at 5000 g for 10 min at 40C. After final washing, the weight of pelleted bacteria was determined and suspended in cold PBS at 100 mg/ml. The suspension was immediately suspended, 1 ml aliquot in eppendrof tubes and stored at 1000C. Care was taken not to refrigerate the aliquots once they were thawed. The aliquot was used in the estimation of nitrite and nitrate (NOx) concentration.
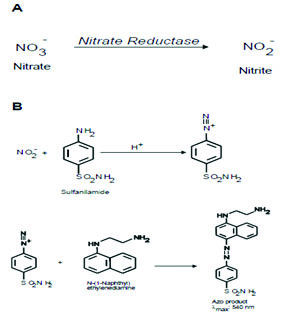
Absorbances were read at 545 nm. Concentration was calculated from the standardization curve of nitrite in μM/ltr.
Estimation of Nitrothiol in Serum by Cook Method [
15]
Principle: The S-NO˙ bond is broken by metal ions like Hg2+ to release NO. The released NO˙ reacts with Griess reagent to form coloured chromophore. Absorbance measured at 496 nm. To avoid interference by nitrite, if present the reaction is not carried out at acidic pH.
Calculations were done using absorptivity. It was measured in μM/ltr.
ε496 = 11500 M-1 cm-1
Estimation of Total Thiol by Habeeb Method [
16]
Principle: The proteins are denatured by Sodium Dodecyl Sulphate (SDS) and urea. SDS also dissolves the membranes. Thus, all the –SH groups present in the mitochondrial proteins namely –SH group which are easily accessible and those present within the proteins are exposed which gives total thiol concentration.
A 5,5’-Dithiobis (2-nitrobenzoic acid) also known as DTNB or Eliman’s reagent reacts with –SH group to form 2-nitro-S-thiobenzoate (NTB) which is yellow coloured compound. The absorbance is read at 412 nm.
Calculations were done using absorptivity. It was measured in μM/ltr.
ε412 = 13600 M-1 cm-1
Results
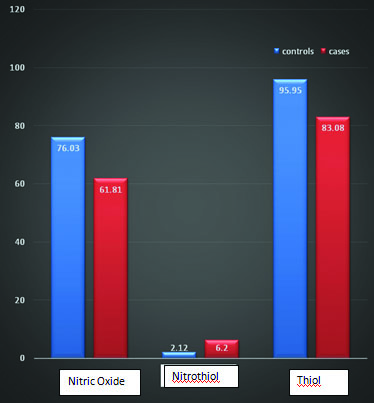
For nitric-oxide, among the controls, mean±SD was 76.03±4.89, in cases 61.81±5.35 (t-test =15.05, p<0.001). Nitrothiol levels in controls and cases were 2.12±0.53 and 6.20±1.47 respectively (t-test= -20.02, p<0.001). Thiol levels in controls and cases were 95.95±4.03 and 83.08±4.03 (t-test= 17.33, p<0.001) [Table/Fig-1].
Indicates nitric oxide, nitrothiol and thiol levels in cases and controls (the mean values).
Discussion
PIH is associated with plethora of biochemical changes. Oxidative stress is one of them and most important change. Recently, nitric-oxide and Reactive Nitrogen Intermediates (RNIs) have been implicated in many pathological conditions. This study was a trial to evaluate the role of both ROIs and RNIs in PIH. In this study, plasma NOx as marker of NO˙ synthesis, nitrothiol, thiol were studied. Oxidative stress reflects an imbalance between the formation of oxidative substances and innate antioxidants that make up the endogenous defence system. Oxidative substances are often free radicals and peroxides. Covalent modification and nitration of proteins and DNA occurs in PIH.
Nitric oxide, known as the ‘endothelium-derived relaxing factor’, or ‘EDRF’, is synthesized endogenously from L-arginine, oxygen and NADPH by various NOS isoenzymes [17]. [Table/Fig-1] shows decreased NOx levels in PIH patients as compared to control (p<0.001). The decrease in mean of PIH was about 14.22 times that of control. Decreased NOx levels directly suggest hypertensive condition. The controversy in different studies may be partly due to the potential confounders like the dietary intake of nitrate. Around 85% of dietary nitrate is derived from vegetables and most of remaining from drinking water. These factors affect the blood level and urinary excretion of NOx. Nitrate content of vegetables is influenced by environmental, agricultural and genetic factors. The NOx levels are also influenced by acute fluctuations in their renal tubular reabsorption [18,19]. However, as mentioned earlier, the present study shows that NOx levels were decreased and hence NO˙ synthesis may be decreased in PIH or its metabolic conversion may be increased leading to decreased flux of NO˙ towards NOx.
Nitrothiol is formed when thiol reacts with peroxynitrite radical. The peroxynitrite radical is formed by reaction between NO˙ and O2 [20,21]. Nitrothiol also undergoes decomposition in presence of appropriate reductants to form NO˙. Thus, nitrothiols are also potent vasodilators whose action is commonly associated with their ability to release NO˙ in physiologically specified locations. [Table/Fig-1] shows that PIH patients have significantly higher levels of S-nitrothiols than normal pregnancy (p<0.01). The difference of mean between cases and control was 4.08. As nitrothiols synthesis involves reaction between NO and O2, thus its concentration is directly proportional to the concentration of NO. However, the present study shows that nitrothiol increased in PIH cases as compared to control. By observing above result the question arises as to why nitrothiol concentration increased when NO concentration is decreased. The possible reason for this could be as follows, there may be increased synthesis of O2 which will scavenge more and more NO˙ towards formation of ONOO-. In this situation, it is possible that in PIH NO˙ synthesis may not be decreased but as more and more NO˙ is used up in reaction with O2, less of it will be oxidized to nitrite and nitrate.
Thus, NOx will be less which will be interpreted as decreased synthesis of NO˙. As more ONOO- is generated, more thiols will be converted to nitrothiol. Hence, nitrothiol concentration is more and NOx concentration is less than control.
Among all the antioxidants that are available in the body, thiols constitute the major portion of the total body antioxidants and they play a significant role in defence against reactive oxygen species [22]. [Table/Fig-1] reflects decreased thiol in PIH as compared to control (p<0.001). Difference in mean value of cases and control is 12.87. Reduced thiol levels suggest the presence of oxidative stress in PIH. Under oxidative stress conditions there is modification of thiol. Many of the changes in thiols, act to protect the protein from oxidative damage. Protection from oxidative damage can occur because the thiol moiety reacts rapidly with radical species, to generate a thiyl radical, protecting other local amino acid residues [23,24]. The sulfhydral groups oxidized and the free radicals are neutralized. In this process, the hydrogen atoms of sulfhydral groups are lost and disulfide bonds are formed between two sulphur atoms [25]. Thus, concentration of thiol decreases.
Thus, the entire scenario in PIH cases summarized as, normally NO˙ is rapidly converted to nitrite (NO2–) and then to nitrate (NO3–). When more and more superoxide radicals formed, nitric-oxide is directed towards formation of peroxynitrite radicals, thus decreasing NO˙ flux towards NOx. Peroxynitrite increases the nitrosative stress. These peroxynitrite radicals react with thiol to form nitrothiol, thus decreasing thiol levels and increasing nitrothiol levels in PIH.
Limitation
The limitation of the present study was that the sample size was less. More elaborative studies with large study population are needed. Some clinical trials regarding use of antioxidants also prove useful.
Conclusion
Thus, it is concluded from this study that nitrosative stress represents a point of convergence for several contributing factors potentially leading to the clinical manifestations of pregnancy induced hypertension. Adaptive mechanisms enhancing the maternal antioxidant defence system that counteract the effects of free radicals through enzymatic induction could prevent the occurrence of nitrosative stress.