Case Report
A 45-year-old male presented to Medicine OPD with history of fever, right sided chest pain and cough for last 20 days. The fever was associated with cough and difficulty in breathing and not associated with chills and rigors. There was no history of evening rise of temperature and night sweats. Chest pain involved anterior and lateral aspect of right side chest. This pain was aggravated on coughing or lying to right lateral side.
Patient had abdominal distension for last seven days which was insidious in onset, gradually progressive and was associated with constipation and colicky abdominal pain. There was also history of difficulty in breathing since, 15 days, which was present only on exertion. There was no history of orthopnoea. Patient was a smoker having 1-2 packs of cigarettes everyday for last 25 years.
On clinical examination patient had left cervical lymphadenopathy involving upper deep cervical nodes, which were 2×2 cm in size, non-tender and not fixed to the skin. Chest examination revealed stony dull note on right side with decreased breathing sounds. Per abdomen examination revealed hepatomegaly with liver palpable 1 cm below the costal margin. A provisional diagnosis of pleural effusion with hepatomegaly was made.
Chest X-ray showed right massive pleural effusion with homogenous opacities of right side of chest, right hilar mass. There was no mediastinal shift. Ultrasound abdomen showed massive hepatomegaly with altered echo texture without parenchymal disease. Contrast Enhanced Computed Tomography (CECT) chest showed ill defined heterogeneously enhancing mass lesion of soft tissue, dimension 6.6×7.2×7 cm in right peri-hilar region and mediastinal area crossing right bronchus approximately 1.3 cm from carina. It was also encircling right pulmonary artery and superior vene cava. Right sided collapse consolidation was noticed.
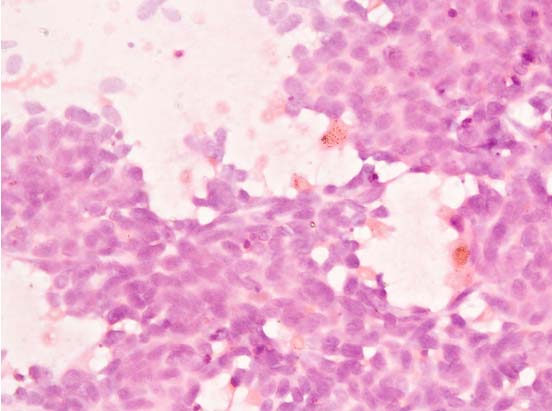
Fine Needle Aspiration Cytology (FNAC) from left cervical lymph node showed clusters, sheets and scattered tumor cells with granular chromatin, inconspicuous nucleoli and barely discernible cytoplasm. Streaking of nuclear material was seen prompting the impression of metastatic small cell carcinoma. The final diagnosis of carcinoma lung with lymph node and liver metastasis was made [Table/Fig-1].
FNAC of cervical lymph node showing clusters of scattered tumor cells with granular chromatin, inconspicuous nucleoli and barely discernible cytoplasm (400X).
Under aseptic conditions right sided diagnostic pleural tap was done. A 20 ml of dark straw colored fluid was aspirated and sent for investigations. Smear from pleural fluid showed lymphocytes, neutrophils, macrophages and reactive mesothelial cells. No malignant cells were seen. It was negative for acid fast bacteria.
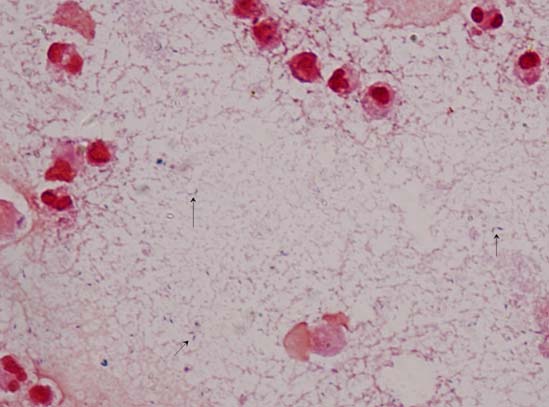
Gram stain of the pleural fluid showed pus cells and gram-positive bacilli [Table/Fig-2]. On 5% sheep blood agar there was one type of growth which was small (2-3 mm), non-haemolytic, convex, smooth colonies with entire edge. Gram staining of the colonies showed gram-positive bacilli. Other biochemical tests included negative catalse, absence of hydrogen sulfide formation in Triple Sugar Agar (TSA) and negative nitrate reduction. The presumptive diagnosis was made as Lactobacillus species according to standard microbiological procedures [1]. Later the identification of the isolate was based on the protein profile using Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS, Bruker Daltonics, Germany). Spectra were analysed using MALDI Biotyper 3 and the isolate was identified as Lactobacillus coryniformis with a high score of 2.2. This isolate was sensitive to chloramphenicol, penicillin, vancomycin, erythromycin, doxycycline, teicoplanin, gentamycin and resistant to ciprofloxacin, clindamycin and co-trimoxazole according to CLSI criteria [2]. Additionally, second sample of pleural sample grew the same bacterial isolate and showed the same sensitivity.
Gram stain of the pleural fluid showed pus cells and gram-positive bacilli (1000X).
Patient was initially managed with vancomycin (500 mg i.v. every six hourly) and dexamethasone (4 mg i.v. every 12 hour). Patient had mild clinical improvement and patient was started on chemotherapy.
Discussion
Members of the family Lactobacillaceae are the normal flora of the human oropharynx, gastrointestinal tract and female genital tract [3]. These gram-positive bacilli are also widely distributed in water, sewage and food items like dairy products, meat, fish and grains [4]. Lactobacillus species mostly causes opportunistic infections in immunocompromised individual due to its low virulence. The common infections caused by them are septicaemia with or without endocarditis and rarely localised infections like gynaecologic and pleuro-pulmonary infections [5]. These infections are more likely to occur in patients with underlying immunosuppressive conditions like bone marrow transplant, diabetes mellitus, and neutropenia, use of broad spectrum antibiotics or history of invasive gastrointestinal or respiratory tract instrumentation [6]. We reported a case of pleuro-pneumonia caused by Lactobacillus coryniformis in a patient with metastatic small cell carcinoma and review the literature of Lactobacillus species causing pleuro-pneumonia infections.
Published cases of pulmonary infections due to Lactobacillus species were identified by a Medline search. In addition, we included references of the past case reports identified in any of the reviewed articles. The search term was ‘Lactobacillus, pulmonary infections’ and search was limited to English language. An effort was made to acquire the original publication in each case.
Since, lactobacilli resemble diptheroids as both are gram-positive bacilli, the following case definition was used: repeated isolation of Lactobacillus species from clinical sample in pure culture, direct microscopic identification in infected sample, and exclusion of other causes. Other than this clinical and microbiological correlation was must.
The following data was reviewed from each of the case: year of isolation, patients’ age, gender, clinical sample tested, species of Lactobacillus, susceptibility of Lactobacillus species, concomitant organisms identified, risk factors/ patient’s co-morbidity and the patient outcome. The microbiological methods used to isolate and identify the Lactobacillus species were not evaluated.
In the literature searched, 14 cases of lactobacilli pleuro-pneumonia have been reported since the first case in 1982 [7]. Out of these 14 reported case, gender and sex distribution was available for only 11 cases [8–16]. There were 7 males and 4 females with documented lactobacilli pleuro-pneumonia. The average age of the patient was 42 years (range 11 months–61 years).
The samples from which Lactobacillus species were isolated ranged from sputum (n-7) [7,9–11,14], Bronchoalveolar lavage (n-5) [8,10,12,15,16] and pleural fluid (n-2) [3,4]. Additionally in one patient Lactobacillus was isolated from both BAL and transthoraic needle aspirate as shown in [Table/Fig-3].
Patient characteristics from various cases of Lactobacillus pleuro-pneumonia.
| Year, author | Bacterial isolate | Sex | Age | Sample | Additional bacterial isolate growing in the sample | Risk factor | Blood culture | Outcome |
|---|
| 1982, Rahman M et al., [7] | Lactobacillus casei ss rhamnousus | Female | 61 years | Sputum | - | Chronic myeloid leukemia | Negative | Died |
| 1989, Querol JM et al., [8] | Lactobacillus species | Male | 40 years | Transthoracic needle aspiration (TNA), bronchoscopy sample | - | Trachea-oesophageal fistula secondary to oesophageal carcinoma | Positive | Died |
| 1989, Querol JM et al., [8] | Lactobacillus species | - | - | Pleural fluid | Bacteroides distansonis | Squamous carcinoma of oesophagus | Negative | Died |
| 1989, Querol JM et al., [8] | Lactobacillus species | - | - | Pleural fluid | Pseudomonas aeruginosa | Hepatic cirrhosis, surgical repair of oseophageal varices | Negative | Died |
| 1992, Namnyak SS et al., [9] | Lactobacillus casei ss rhamnosus | Male | 73 years | Sputum | - | Lung abscess secondary to chronic emphysema | Negative | Died |
| 1993, Sriskandan S et al., [10] | Lactobacillus casei ss rhamnosus | Male | 4 years | Sputum | - | Bone marrow transplant | Negative | Died |
| 1993, Sriskandan S et al., [10] | Lactobacillus fermentum | Male | 46 years | Sputum | - | AIDS | Negative | Died |
| 1993, Sriskandan S et al., [10] | Lctobacillus acidophilus | - | - | BAL | Saccharomyces cerevisiae | Immunosuppression due to vaculitis | Negative | Died |
| 1994, Jones SD et al., [11] | Lactobacillus species | Male | 52 years | Sputum | - | lung transplantation | Negative | Recovered |
| 1997, Fruchart C et al., [12] | Lactobacillus paracasei | Male | 63 years | Bronchoalveolar lavage (BAL) | - | Neutropenia | Negative | Recovered |
| 1997, Abgrall S et al., [13] | Lactobacillus casei ss rhamnosus | Female | 38 year | Bronchoalveolar lavage (BAL) | - | AIDS, | Negative | Died |
| 1998, Rogasi PG et al., [14] | Lactobacillus casei ss rhamnosus | Male | 45 years | Sputum | - | AIDS | Negative | Recovered |
| 2002, Wood GC et al., [15] | Lactobacillus species | Female | 39 years | Bronchoalveolar lavage (BAL) | Methicillin resistant Staphylococcus aureus (MRSA) | Diabetes, Crohn’s Disease | Negative | Recovered |
| 2014, Doern CD et al., [16] | Lactobacillus rhamnosus | Female | 11 months | Bronchoalveolar lavage (BAL) | - | Trisomy 21 with RSV pneumonis taking probiotics supplement | Negative | Recovered |
Among the 14 lactobacilli isolates, 9 were further identified till species level. Four different species were isolated; Lactobacilluscasei ss rhamnosus (n-5), Lactobacillusfermentum (n-1), Lactobacillusacidophilus (n-1) and L. paracasei (n-1).
Pure growth of lactobacilli was seen in 10 out of the 14 cases of lactobacilli pleuro-pneumonia. In the remaining 4 cases, there was growth of second pathogen along with Lactobacillus. The accompanying pathogens were Bacteroides distansonis, Pseudomonas species, Saccharomyces cerevisiae, and methicillin resistant Staphylococcus aureus respectively as shown in [Table/Fig-3].
Of the 14 patients who presented with pleuro pneumonia infection, only one patient had blood culture positive for lactobacilli species [17]. This patient also had trachea-oesophageal fistula secondary to oesophageal carcinoma and bronchopneumonia was probably the focus of haematogenous spread.
The majority of the isolates showed susceptibility to ampicillin (100%), gentamycin (100%), erythromycin (100%), clindamycin (66.6%) and tetracycline (66.6%) [Table/Fig-4]. Only 5 isolates were tested for vancomycin and among them one isolate showed sensitivity to it. One Lactobacillus isolate was resistant to vancomycin but sensitive to teicoplanin. Additionally decreased sensitivity was seen to ceftriaxone (33.3%). None of the strains was sensitive to co-trimoxazole, amikacin, pefloxacin and tobramycin.
Antimicrobial susceptibility of Lactobacillus species isolated from various pleuro-pulmonary infections.
| Antimicrobial agent | No. of isolates tested | No. of isolates susceptible (%) |
|---|
| Penicillin | 10 | 6 (60%) |
| Ampicillin | 4 | 4(100%) |
| Clindamycin | 6 | 4(66.6%) |
| Cotrimoxazole | 3 | 0(0%) |
| Tetracycline | 3 | 2(66.6%) |
| Gentamycin | 4 | 4(100%) |
| Amikacin | 1 | 0(0%) |
| Ciprofloxacin | 4 | 2(50%) |
| Pefloxacin | 1 | 0(0%) |
| Erythromycin | 5 | 5(100%) |
| Ceftriaxone | 3 | 1(33.3%) |
| Tobramycin | 2 | 0(0%) |
| Vancomycin | 5 | 1(20%) |
| Teicoplanin | 1 | 1(100%) |
All the patients had severe associated co-morbidities. The most common risk factor was immunosuppresion which was present in 7 out of 14 patients (50%) [10–14]. Additionally 21.5% of patients had carcinoma (3/14), another 21.5% of the patients had chronic disease (3/14) [15–17]. Among the immunosuppresion AIDS was more commonly seen [13,14]. One patient had Lactobacillus pneumonia linked to consumption of probiotics supplement. Out of the seven patients with immunosuppresion, three patients had AIDS, two patients were on immunosuppressive agents following organ transplant, one patient was neutropenic and one patient had history of intake of immunosuppressive drugs for long standing vasculitis (as shown in [Table/Fig-3]).
The portal of entry could be established in only 7 out of 14 patients. In four patients the documented route of entry was through the gastrointestinal tract [7,8,17]. In one patient the probable route of entry of Lactobacillus bacteria was via the transplanted lung [11]. Another immunocompetent patient had ventilator associated pneumonia due of Lactobacillus species [15]. This patient’s major risk factors were mechanical ventilation and thoracic trauma. Lastly, in one patient the route of lactobacillus pneumonia was aspiration of probiotics strain of Lactobacillus species [16]. This patient was later diagnosed to be having a trachea-oesophageal fistula.
Outcome of 12 patients could be recorded and 6 patients (50%) died during hospitalization for Lactobacillus pleuro pneumonia [8–10,13]. But attributable mortality couldn’t be ascertained and this was only overall mortality.
All the cases were analysed with regards to epidemiology, risk factors and clinical outcome. Several points in this evaluation merit further discussion. Lactobacillus pulmonary infection remains an unusual clinical entity with poorly defined significance.
The Lactobacillus species can cause a variety of infections but more commonly it causes bacteraemia with or without endocarditis [18,19]. Cannon JP et al., reviewed all Lactobacillus cases retrospectively and found that out of 241 clinically relevant cases, 52.5% (129/241) were of Lactobacillus bacteraemia and only 16% (39/241) were localised Lactobacillus infections [20]. In our review of literature, out of 14 patients with Lactobacillus pleuro- pneumonia infection only one patient had concurrent lactobacillemia. Therefore, Lactobacillius species can be a primary cause of pleuropneumonia without bacteraemia especially in immunocompromised patients.
The clinically significance of isolation of Lactobacillus species from a normally sterile site is debatable [6,21,22]. Pleuro-pulmonary infections due to Lactobacillius species may be under reported because of many reasons. Most species of Lactobacillus are facultative, although certain species grow best under microaerophilic or anaerobic conditions particularly on primary isolation. Additionally, lactobacilli require extended incubation time [23]. Therefore, the isolation and identification of Lactobacillus becomes difficult. Moreover, the morphology of lactobacilli resembles other gram-positive bacilli like Corynebacterium and Clostridium; it may be dismissed as a contaminant [24]. But the microbiologist should be aware that the lactobacilli though rare, can be an important opportunist pathogen causing pleuropneumonia especially if the patients is immunocompromised or has carcinoma or neutropenia. Many studies have evaluated the pathogenic potential of lactobacilli. Lactobacilliusrhamnosus and Lactobacillius paracasei subspecies paracasei possess various virulence factors like production of enzymes which breaks down human glycoprotein, binding to extracellular protein like fibronectin, fibrogen and collagen which may be important in early stage colonization and adherence [25–28]. Also, lactobacilli have the ability to aggregate human platelets, which is considered a significant pathogenic trait in pathogenesis of various infections [25–27].
Lactobacilli pleuro-pneumonia was found to be associated with other serious underlying illness. Various host factors favour lactobacilli pleuro-pneumonia like defective CMI, neutropenia, alteration of normal gastrointestinal mucosal barrier by opportunistic pathogens and administration of broad spectrum anti-microbial [5,11].
Our patient had small cell carcinoma with metastasis. Husni RN et al., reviewed 45 cases of lactobacilli bacteraemia and found that the majority of patients had serious diseases; 48% of the patients were admitted in Intensive Care Unit (ICU), 40% had malignancy, 38% had undergone malignancy and 27% of the patients had diabetes mellitus [19].
Similarly Cannon JP et al., retrospectively studied the pathogenic relevance of lactobacilli and found cancer, diabetes and transplantation to be more common underlying conditions associated with infections with lactobacilli [20]. Therefore, the isolation of Lactobacillus species in patients presenting with pleuropneumonia in association with immunosuppresion or cancer should be taken as significant.
Salminen MK et al., reviewed risk factors of 89 case of lactobacilli bacteraemia and concurred that immunosuppresion, prior hospitalisation; previous antibiotics treatment and surgery were common pre-disposing factors for the same [29].
In four patients the gastrointestinal tract, which is the normal habitat of lactobacilli, was considered to be an important route of entry of lactobacilli to the pulmonary cavity [7,8]. This path from gastrointestinal tract to pleura was postulated to be through a fistula. In other cases surgical procedures on gastrointestinal tract was hypothesised to cause, spread of lactobacilli to pulmonary area. In our patient, dissemination from gastrointestinal tract could be probably the source of infection. This could be favoured by decreased immunity due to metastatic small cell carcinoma. But in recent times, unusual routes have been postulated to spread lactobacilli to pleuro-pulmonary space.
Wood GC et al., describes a case of lactobacilli causing Ventilator Associated Pneumonia (VAP) in a critically ill patient [15]. The various factors which contributed to the development of VAP were mechanical ventilation, thoracic trauma, diabetes mellitus, obesity and smoking. The clinical correlation of lactobacilli isolate with VAP was made because quantitative Broncho-Alveolar Lavage (BAL) culture showed ≥ 105 Cfu/ ml of Lactobacillus species. This study highlights the significance of lactobacilli as a causative agent of VAP in immunocompetent patient on ventilator [15].
Doern CD et al., reported a case of aspiration pneumonia due to probiotics strain; Lactobacillus rhamnosus [16]. Herein an eleven-month-old child with trisomy 21 with RSV infection had taken a probiotics strain “Lactobacillus rhamnosus”, three months prior to her illness. Strain typing of L. rhamnosus strains both probiotics and clinical isolates was performed using repetitive sequence PCR, showed the probiotics and patient’s clinical strain to be identical with a similarity index of > 99%. There have been other confirmed reports of probiotics strain causing blood stream infections, urinary tract infections and peritonitis [30–33]. This report shows how in a susceptible population, a seemingly non-pathogen probiotics strain can cause disease.
Jones SD et al., reported a case of Lactobacillus pneumonia transmitted by transplanted lung [11]. Though only one case has been reported so for but it has greater significance in lung transplant patients. Both donor and recipient should be screened for lactobacilli and if found treated. This bacterium of low virulence assumes greater role in patients who are immunocompromised. Husni RN et al., reported two cases of lactobacillemia in patients with lung transplant [19]. Additionally there have been reports of lactobacillemia following hepatic and heart transplant [34,35].
The most common species was Lactobacillus rhamnosus followed by Lactobacillus fermentum, Lactobacillus acidophilus and L. paracasei. The species isolated in our study was Lactobacillus coryniformis; which is the first time this particular species has been associated with pleuro-pulmonary infection. Similar result was seen by Salminen MK et al., who reported total of 53% cases of bacteraemia due to L. rhamnosus followed by L. fermentum (20%) and then L. casei (15%) [29].
Anti-microbials susceptibility data of lactobacilli species is inadequate because there have been very few studies demonstrating the sensitivity pattern of this bacteria. Further, the testing of clinical isolates from patients has not been tested to a uniform set of antimicrobial agents.
Husni RN et al., in their study of 45 cases of Lactobacillus bacteraemia and endocarditis reported excellent susceptibility of the isolates to penicillin and ampicillin [19]. The susceptibility of various Lactobacillus isolates to various antibiotics from pleuro-pulmonary infection shows that highest sensitivity was to ampicillin (100%) and least to penicillin and ceftriaxone (33%) [Table/Fig-4]. In contrast Sewson JM et al., showed decreased susceptibility to β-lactam like penicillin, ampicillin, cephalothin and ceftriaxone [36]. Sussman JI et al., postulated the reason for low susceptibility of lactobacilli to penicillin and ampicillin could be because of the ability of lactobacilli to produce lactic acid which lowers the pH [5]. The bactericidal activity of ß-lactam is due to an autolytic enzyme, whose activity decreases when the pH is lowered. Therefore, the role of ß-lactam in therapy of lactobacilli infection has to be individualised [5].
Role of vancomycin in treatment of lactobacilli is not clear. Husni R N, reported 27% of 22 isolates to be susceptible to vancomycin [19]. Similarly Cannon JP et al., showed 22.5% of isolates to be susceptible to vancomycin [20]. In our review one isolated out of the five lactobacilli to be tested against vancomycin was susceptible (20%). In addition our isolate Lactobacillus coryniformis was sensitive to vancomycin. In contrast Swenson JM et al., demonstrated that lactobacilli are resistant to vancomycin with high MIC [36].
Swenson JM et al., studied antimicrobial susceptibility of vancomycin resistant lactobacilli and reported them to be highly susceptible to the action of erythromycin, clindamycin, gentamycin, tobramycin and chloramphenicol and decreased susceptibility to ciprofloxacin and trimethoprim-sulphamethazole [36]. Similarly in our review good susceptibility was seen to erythromycin, gentamycin and clindamycin and isolates showed decreased susceptible to ciprofloxacin and trimethoprim-sulphamethazole.
A high overall mortality (nearly 50%) was seen in our review in patients with Lactobacillus pleuro-pneumonia infections. This was overall mortality and not attributable mortality since, all the patients were serious and had poor long term prognosis. Husni RN et al., reviewed 45 cases of Lactobacillus bacteraemia and endocarditis [19]. They found only one death directly attributable to lactobacillemia and observed that lactobacilli exhibit low virulence.
Conclusion
In conclusion, isolation of Lactobacillus species from pleuro- pneumonia infection could be a marker of poor long term prognosis. Clinical microbiologist should be aware about the possibility of this infection and should identify these gram-positive bacilli isolated from patients with suspected pleuro- pneumonia infection. In patients with carcinoma and with immunosuppresion having Lactobacillus isolates from their pulmonary sample should have guarded prognosis. Our patient had metastatic small cell carcinoma which was an important risk factor for Lactobacillus pleuro-pulmonary infections. Also, the diagnosis of these infections requires both microbiologist and clinical correlation to rule out contamination.
[1]. Howard AJ, Ison CA, Haemophilus, Gardnerella and other bacilliIn Mackie and McCartney, Practical Medical Microbiology 1996 14th ednChurchill Livingstone:449-66. [Google Scholar]
[2]. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility; twenty-fifth informational supplement. CLSI document M100-S25 Clinical and laboratory standards institute, Wayne, Pennsylvania, USA. 2015 35:3 [Google Scholar]
[3]. Koneman EW, Allen SD, Janda WM, Schreckenberger PC, WC, Diagnostic microbiology 1992 PhiladelphiaJ.B. Lippincott Company:433-445.:484-486. [Google Scholar]
[4]. Isenberg HD, D’Amato RF, Indigenous and pathogenic micro-organisms of humans. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken edsManual of clinical microbiology 1995 6th edWashington, DCASM Press:5-18. [Google Scholar]
[5]. Sussman JI, Baron EJ, Goldberg SM, Clinical manifestations and therapy of Lactobacillus endocarditis: report of a case and a review of the literatureRev Infect Dis 1986 8:771-76. [Google Scholar]
[6]. Antony S, Lactobacillemia: an emerging cause of infection in both the Immunocompromised and the immunocompetent hostJ Natl Med Assoc 2000 92:83-86. [Google Scholar]
[7]. Rahman M, Chest infection caused by Lactobacillus casei ss rhamnosusBr Med J 1982 284:471-72. [Google Scholar]
[8]. Querol JM, Manresa F, Izquierdo J, Cisnal M, Lactobacillus pneumonia in a patient with oesophageal carcinomaEur Respir J 1989 2:589-91. [Google Scholar]
[9]. Namnyak SS, Blair ALT, Hughes DF, McElhinney P, Donnelly MR, Corey J, Fatal lung abscess due to Lactobacillus casei ss rhamnosusThorax 1992 47:666-67. [Google Scholar]
[10]. Sriskandan S, Lacey S, Fischer L, Isolation of vancomycin resistant lactobacilli from three neutropenic patients with pneumoniaEur J Clin Microbiol Infect Dis 1993 12:649-50. [Google Scholar]
[11]. Jones SD, Fullerton DA, Zamora MR, Badesch DB, Campbell DN, Griver FL, Transmission of Lactobacillus pneumonia by a transplanted lungAnn Thorac Surg 1994 58:887-89. [Google Scholar]
[12]. Fruchart C, Salah A, Gray C, Martin E, Stamatoullas A, Bonmarchand G, Lactobacillus species as emerging pathogens in neutropenic patientsEur J Clin Microbiol Infect Dis 1997 16:681-84. [Google Scholar]
[13]. Abgrall S, Joly V, Derkinderen P, Decré D, Carbon C, Yeni P, Lactobacillus casei infection in an AIDS patient [letter]Eur J Clin Microbiol Infect Dis 1997 16:180-82. [Google Scholar]
[14]. Rogasi PG, Vigano S, Pecile P, Leoncini F, Lactobacillus casei pneumonia and sepsis in a patient with AIDS. Case report and review of the literatureAnn Ital Med Int 1998 13:180-82. [Google Scholar]
[15]. Wood GC, Boucher BA, Croce MA, Fabian TC, Lactobacillus species as a cause of ventilator–associated pneumonia in a critically ill trauma patientPharmacotherapy 2002 22(9):1180-82. [Google Scholar]
[16]. Doern CD, Nguyen SR, Afolabi F, Burnham CA, Probiotics –associated aspiration pneumonia due to Lactobacillus rhamnosusJ Clin Microbiol 2014 52(8):3124-26. [Google Scholar]
[17]. Querol JM, Manresa F, Barbe F, Cisnal M, Lactobacilli and pleuropulmonary infectionEur Resp J 1989 2:1021-22. [Google Scholar]
[18]. Sharpe ME, Hill LR, Lapage SP, Pathogenic lactobacilliJ Med Microbiol 1973 6:281-86. [Google Scholar]
[19]. Husni RN, Gordon SM, Washington JA, Longworth DL, Lactobacillus bacteraemia and endocarditis: review of 45 casesClin Infect Dis 1997 25:1048-55. [Google Scholar]
[20]. Cannon JP, Lee TA, Bolanos JT, Danzinger LH, Pathogenic relevance of Lactobacillus: a retrospective review of over 22 casesEur J Clin Microbiol Infect Dis 2005 24:31-40. [Google Scholar]
[21]. Antony S, Dummer S, Stratton C, Lactobacillus bacteraemia and endocarditisClin Infect Dis 1998 26:1483-84. [Google Scholar]
[22]. Antony SJ, Stratton CW, Dummer JS, Lactobacillus bacteraemia: description of the clinical course in adult patients without endocarditisClin Infect Dis 1996 23:773-78. [Google Scholar]
[23]. Murray PR, Baron E, Jorgenson JH, Pfaller MA, Yolken RH, Manual of clinical microbiology 2003 Washington DCASM Press:857:869 [Google Scholar]
[24]. Gallemore GH, Mohon RT, Ferguson DA, Lactobacillus fermentum endocarditis involving a native mitral valveJ Tenn Medical Assoc 1995 88:306-08. [Google Scholar]
[25]. Oakey HJ, Harty DWS, Know KW, Enzyme production by lactobacilli and the potential link with infective endocarditisJ Appl Bacteriol 1995 78:142-48. [Google Scholar]
[26]. Harty DWS, Oakey HJ, Patrikakis M, Hume EBH, Knox KW, Pathogenic potential of lactobacilliInt J Food Microbiol 1994 24:179-89. [Google Scholar]
[27]. Harty DWS, Patrikakis M, Knox KW, Identification of Lactobacillus strains from patients with infective endocarditis and comparison of their surface-associated properties with those of other strains of the same speciesMicrob Ecol Health Dis 1993 6:191-201. [Google Scholar]
[28]. Harty DWS, Patrikakis M, Hume EBH, Oakey HJ, Knox KW, The aggregation of human platelets by Lactobacillus speciesJ Gen Microbiol 1993 139:2945-41. [Google Scholar]
[29]. Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, Lactobacillus bacteraemia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GGClin Infect Dis 2004 38:62-69. [Google Scholar]
[30]. Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK, Lactobacillus sepsis associated with probiotic therapyPediatrics 2005 115:178-81. [Google Scholar]
[31]. Kunz AN, Noel JM, Fairchok MP, Two cases of Lactobacillus bacteraemia during probiotic treatment of short gut syndromeJ Pediatr Gastroenterol Nutr 2004 38:457-58. [Google Scholar]
[32]. Mackay AD, Taylor MB, Kibbler CC, Hamilton-Miller JM, Lactobacillus endocarditis caused by a probiotic organismClin Microbiol Infect 1999 5:290-92. [Google Scholar]
[33]. Kochan P, Chmielarczyk A, Szymaniak L, Brykczynski M, Galant K, Zych A, Lactobacillus rhamnosus administration causes sepsis in a cardio surgical patient—is the time right to revise probiotics safety guidelines?Clin Microbiol Infect 2011 17:1589-92. [Google Scholar]
[34]. Patel R, Cockerill FR, Porayko MK, Osmon DR, Ilstrup DM, Keating MR, Lactobacillemia in liver transplant patientsClin Infect Dis 1994 18:207-12. [Google Scholar]
[35]. Toporoff B, Rosado LJ, Appleton CP, Sethi GK, Copeland JG, Successful treatment of early infective endocarditis and mediastinitis in a heart transplant recipientJ Heart Lung Transplant 1994 13:546-48. [Google Scholar]
[36]. Swenson JM, Facklam RR, Thornsberry C, Antimicrobial susceptibility of vancomycin resistant Leuconostoc, Pediococcus, and Lactobacillus speciesAntimicrob Agents Chemother 1990 34:543-49. [Google Scholar]