Ovarian carcinoma represents 30% of all cancer cases of the female genital tract. Among them SEOC constitute more than 90% of the malignant neoplasm arising in the ovary and is the most lethal gynecologic malignancy [1–3].
Although ovarian carcinoma is ten times less common than carcinoma of the breast, it is associated with advanced stage where the disease is extended beyond the structures of the pelvis in 70–75% of patients. The incidence of recurrence after surgery and chemotherapy is high with more number of deaths in due course of disease [3,4].
Efforts at early detection and new therapeutic approaches to reduce mortality have been mainly ineffective due to absence of definitive aetiological factors and diagnostic aids for screening.
Multiple factors such as age, race, histologic type, grade, FIGO stage, residual disease, CA125 levels and performance status at the time of diagnosis influence survival of SEOC.
These factors failed to explain the biological behaviour of ovarian cancer and hence, more objective ways to establish the prognosis are needed [5–8].
Cell proliferation plays an important role in the clinical behaviour and aggressiveness of ovarian carcinoma. Determination of proliferative activity has been reported to be of a diagnostic with prognostic value and many methods are used to estimate the number of proliferating cells [7–10].
Ki-67 antigen immunostaining to determine proliferation of malignant cells is one such reliable prognostic marker and is a relatively new technique for estimating the Proliferative Index (PI) of a neoplastic lesion by the Mindbomb E3 ubiquitin protein ligase1 (MIB-1 antibody) Immunohistochemistry (IHC). Ki-67 antigen is over expressed in malignant ovarian tumour compared to benign or borderline tumours of surface epithelial origin. High expression of Ki-67 antigen is associated with tumour aggression, vascular invasion, tumour metastasis, reserved prognosis and poor response to chemotherapy. Its expression can be used to guide the clinical management of ovarian carcinoma both as diagnostic and prognostic tool [2,3,9,11–13].
In the present study, we correlated the Ki-67 antigen expression with histological subtype and grade, FIGO staging of SEOC, preoperative CA125 level and investigate the clinical value of Ki-67 antigen as a proliferative marker for diagnostic and prognostic purpose.
Materials and Methods
It was a descriptive cross-sectional study conducted in JSS Medical College and Hospital, JSS University from August 2013 to July 2015, Mysuru, Karnataka, India involving 40 cases of SEOC over a period of two years. All the cases were evaluated after obtaining approval from the institutional ethical committee with informed consent from the patients. All specimens were received in 10% formalin with relevant clinical information and preoperative CA 125 level were documented from the medical records. The specimens were then subjected to gross description and adequate sampling by representative tissue section for routine histopathological process. The microscopic features were studied with Haematoxylin and Eosin (H&E) staining of 4 μm thick sections obtained from representative paraffin blocks. Histological grading was done using the Universal Grading System. All grade 2 and 3 tumours were considered as high grade tumours and a two tier grading system was used for serous carcinoma as High Grade Serous Carcinoma (HGSC) and Low Grade Serous Carcinoma (LGSC). FIGO stage at the time of diagnosis was calculated based on preoperative radiological, operative and histopathological findings. Ki-67 antigen immunostaining was performed on all 40 cases. Antigen retrieval was done with pressure cooked method using sodium citrate buffer (0.01 M; pH 6.0) on 4 μm thick tissue sections. MIB-1 antibody/ monoclonal antibody (Novocastra, code no Ki-67-MM1-R7-C) from Novostain Universal Detection Kit (Novocastra, code no. RTU- Ki-67-MM1) was used for Ki-67 antigen detection by standard streptavidin- biotin technique.
Reactive lymph node sections were taken as positive control whereas, sections treated with tris-buffer solution alone without primary antibody was used as negative control. A brown granular nuclear reactivity was interpreted as positive.
Immunostaining was assessed qualitatively by two pathologists at low power examination in the fields consisting of regions of the tumour having the greatest number of immunoreactive cells, where an uneven distribution of immunohistochemical labeling was evident, the area of maximal labeling fields was chosen for counting. Quantitatively the PI was expressed as Ki-67 LI/MIB-1 labeling index when percentage of positively stained cells per 100 epithelial cells after counting at least 1000 cells in each case using high power objective of the microscope (x400) [12,13]. After going through several studies, the Ki-67 LI was grouped as high LI (>50% immunoreactive cells are positive) and low LI (<50% immunoreactive cells are positive) as there is no international consensus on cutoff value for percentage of expression [6,9,10,12–16]. The staining pattern was interpreted as: a) Focal pattern: a small number of positively stained cells; b) diffuse pattern: uniform diffuse positively stained cells; and c) heterogenous pattern: areas of strong positively stained cells alternating with areas of low positively stained cells.
Statistical Analysis
All the statistical methods were carried out through the SPSS for Windows (version 16.0). A p-value of ≤ 0.05 was taken to be statistically significant. Descriptive statistics such as mean, standard deviation, and percentage along with Chi-square test were used to know the association.
Results
Among the 40 cases highest incidence of SEOC was noticed in the 61 to 70 years group, with a mean age of 54.72±10.9 years [Table/Fig-1]. Also it was observed that most of the cases of serous carcinomas were above the age of 50 years. Majority of the patients (45%) presented with abdominal discomfort/distension at the time of diagnosis. In terms of location, 62.5% had unilateral involvement and grossly 70% were solid in consistency. Among the bilateral group, serous carcinoma was the most common morphologic type.
Clinicopathological characteristics of 40 surface epithelial ovarian carcinoma cases.
| Characteristics | No. of patients (%) |
|---|
| 1) | Age at diagnosis (years) | 40(100%) |
| 2) | Chief complaint | |
| Abdominal distension | 18 (45) |
| Abdominal pain | 09 (22.5) |
| Menstrual irregularities | 07 (17.5) |
| Abdominal mass | 05 (12.5) |
| Dyspnea | 01 (2.5) |
| 3) | Gross consistency of the tumours | |
| Solid | 28 (70) |
| Solid and cystic | 11 (27.5) |
| Cystic | 01 (2.5) |
| 4) | Laterality | |
| Unilateral | 25 (62.5) |
| Bilateral | 15 (37.5) |
| 5) | Histological type | |
| Serous carcinoma (SC) | 26 (65) |
| Mucinous carcinoma (MC) | 05 (12.5) |
| Clear cell carcinoma (CC) | 04 (10) |
| Undifferentiated carcinoma (UC) | 04 (10) |
| Transitional cell carcinoma (TC) | 01 (2.5) |
| 6) | Histological grade | |
| Low grade | 10 (25) |
| High grade | 30 (75) |
| 7) | Serous carcinoma | |
| Low grade serous carcinoma | 06 (23.08) |
| High grade serous carcinoma | 20 (76.92) |
| 8) | Ki -67expression | |
| Positive | 40 (100) |
| Negative | 00 (00) |
| 9) | FIGO Stage‡ with subtypes** | |
| SC | MC | CC | UC | TC | |
| Stage I | 5 | 3 | 1 | 1 | 0 | 10 (25) |
| Stage II | 2 | 0 | 2 | 1 | 0 | 05 (12.5) |
| Stage III | 18 | 2 | 1 | 2 | 0 | 23 (57.5) |
| Stage IV | 1 | 0 | 0 | 0 | 1 | 02 (5) |
| 10) | CA 125 levels | 40(100%) |
‡FIGO-International Federation of Gynecology and Obstetrics. **Serous carcinoma (SC), Mucinous carcinoma (MC), Clear cell carcinoma (CC), Undifferentiated carcinoma (UC) Transitional cell carcinoma (TC)
On classification based on histologic type of tumour, serous carcinoma was the common tumour (65%) encountered and no case of endometroid carcinoma documented in this study.
Majority of the tumours (75%) were high grade and within the serous group, 76.92% were HGSC. At the time of diagnosis 57.5% of the cases belonged to FIGO stage III. The mean CA 125 level was found to be 487.49 U/ml.
Ki-67 expression was seen in all 40 cases-100% and Ki-67 LI compared with clinicopathologic variables [Table/Fig-2]. Amongst histological subtypes [Table/Fig-2], the mean Ki-67 LI was highest in serous carcinoma (65.03±21.67) and least in transitional cell carcinoma (38). Ki-67 LI did not show any significant association within the subtypes (p=0.65). There was a statistically significant (p<0.001) higher Ki-67 LI associated with the high grade tumours (mean: 69.9±14.4) as compared to low grade tumours (mean: 39.2±18.9).
Distribution of Ki-67 LI (MIB-1) staining expression according to the clinicopathological variables of 40 patients of surface epithelial ovarian carcinoma.
| Variable | Ki- 67 LI: percentage of cells staining Median | p -value |
|---|
| 1) | Histologic types | | (p=0.65) |
| Serous carcinoma | (65.03±21.67) |
| Mucinous carcinoma | (60.24±21.91) |
| Clear cell carcinoma | (53.35±20.86) |
| Undifferentiated carcinoma | (61.07±9.46) |
| Transitional carcinoma | (38) |
| 2) | Serous and non-serous group | | (p=0.17) |
| Serous group | 65.03±21.67 |
| Non serous group | 53.17±13.16 |
| 3) | Histologic grade | | (p<0.001) |
| High grade tumours | (Mean: 69.9±14.4) |
| Low grade tumours | (Mean: 39.2±18.9) |
| 4) | Serous carcinoma | | (p=0.001) |
| High grade serous carcinoma | (65.34) |
| Low grade serous carcinoma | (37.96) |
| 5) | FIGO Stage† | | (p<0.001) |
| Stage I | 39.7 |
| Stage II | 69.5 |
| Stage III | 70.6 |
| Stage IV | 59.6 |
| 6) | CA 125 levels | – | (p=0.862) |
| No significant correlation |
†FIGO- International Federation of Gynecology and Obstetrics.
The mean Ki-67 LI of serous group was 65.03±21.67 which was significantly higher than the mean Ki-67 LI in non serous group which was found to be 53.17±13.16. Also, the staining pattern of serous group was more diffuse or heterogenous when compared to the non serous groups. Among the serous group, the Ki-67 LI was higher in the HGSC (65.34%) with a strong diffuse pattern of staining [Table/Fig-3a,c,e,g and j], as compared to the LGSC (37.96%) which showed moderate heterogenous staining [Table/Fig-3a,c,e,g and j]. This difference was found to be statistically significant (p=0.001).
a: Low grade serous carcinoma showing confluent papillary pattern lined by tumour cells. (H&E, x40).
b: Low grade serous carcinoma displaying a heterogenous staining pattern with low Ki-67 LI (Ki-67 IHC, x40).
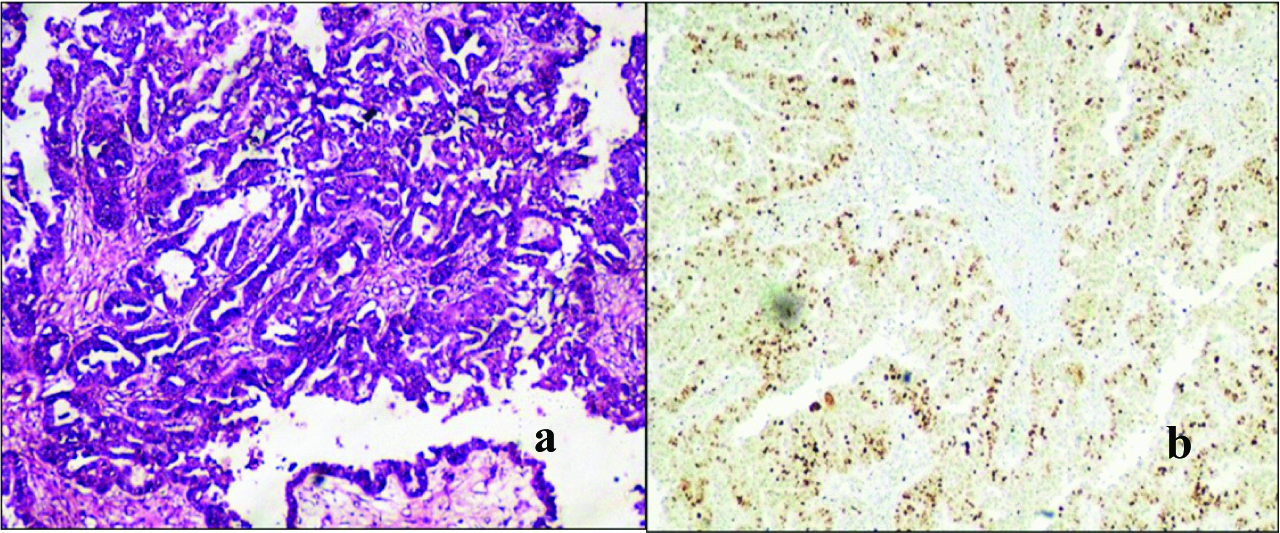
c: High grade serous carcinoma showing confluent papillary pattern lined by tumour cells (H&E, x40).
d: High grade serous carcinoma displaying a diffuse staining pattern of tumour cells with high Ki-67 LI. (Ki-67 IHC, x 40).
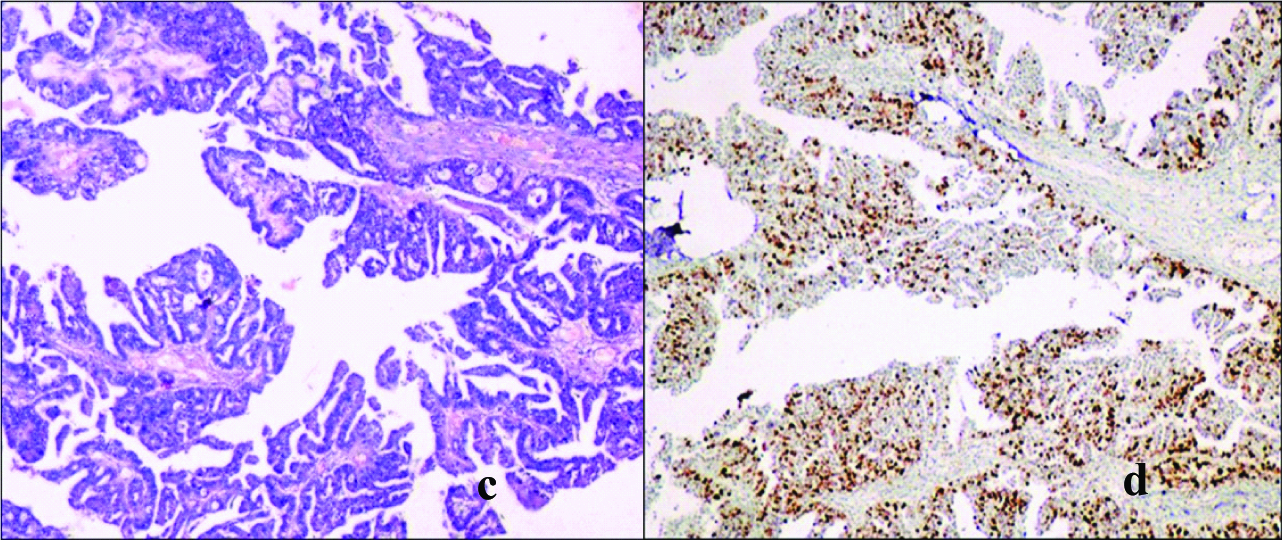
e: High grade mucinous cystadenocarcinoma, intestinal type showing stratified columnar epithelium with hyperchromatic nuclei. (H&E, x40).
f: High grade mucinous cystadenocarcinoma displaying high Ki-67 LI. (Ki-67 IHC x20).
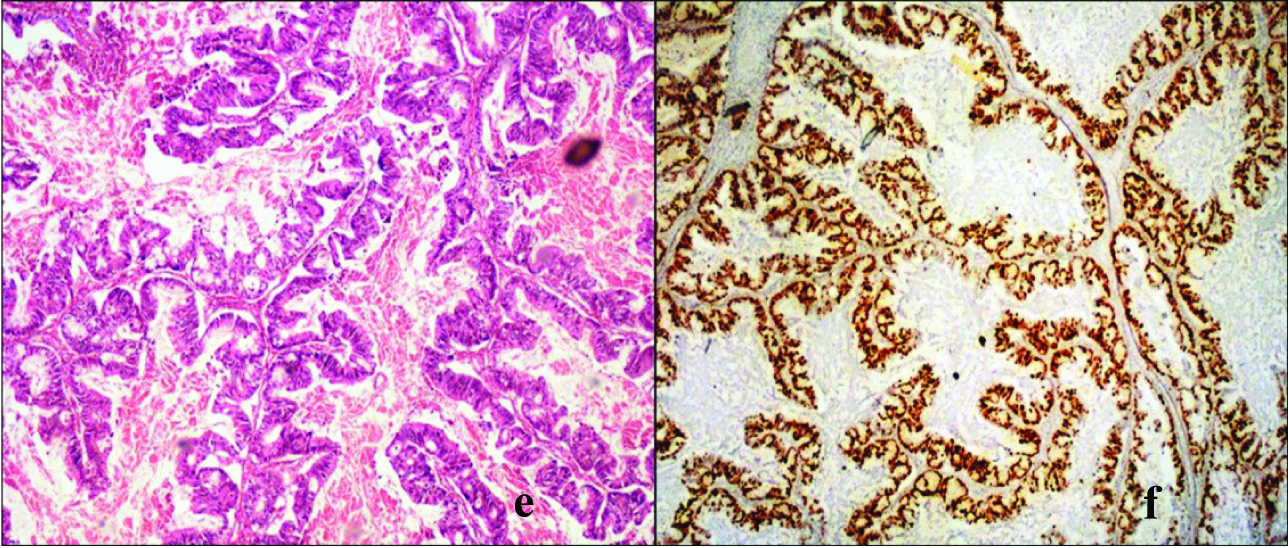
g: High grade clear cell carcinoma, tumour cells with clear cytoplasam and eccentric angular nuclei. (H&E, x200).
h: High grade clear cell carcinoma showing high Ki-67 LI. (Ki-67 IHC, x200).
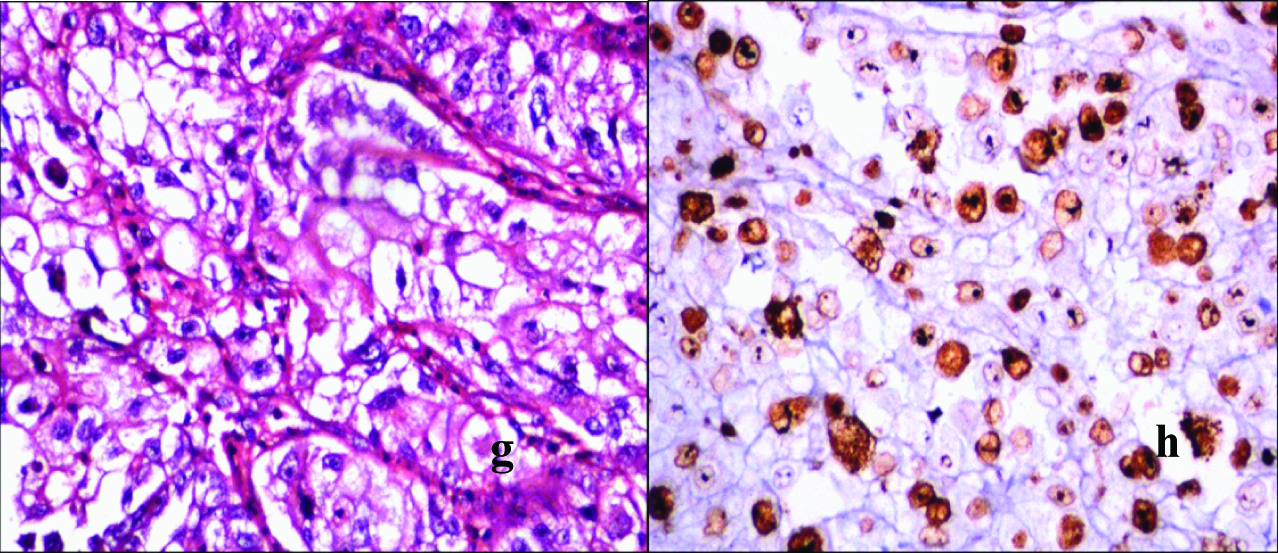
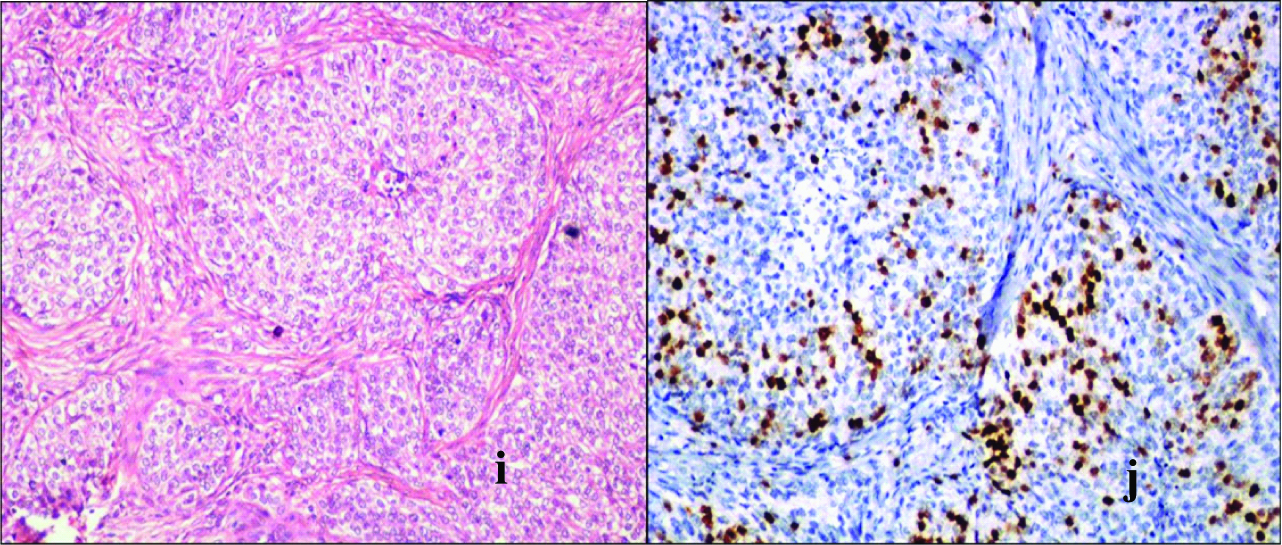
i: Transitional cell carcinoma showing nests and islands of malignant transitional cells infiltrating desmoplastic stroma. (H&E, x40).
j: Transitional cell carcinoma showing low Ki-67 LI. (Ki-67 IHC, x100).
High labeling index of Ki-67 was noted with advanced FIGO stage (stage III-70.6) which was statistically significant (p<0.001). CA 125 levels were significantly raised in serous carcinomas (mean -614.73 U/ml). However, there was no significant correlation between Ki-67 LI and CA 125 levels (p=0.862).
Discussion
Presently, based on histopathology, IHC and molecular genetic analysis, the common types of SEOC are: HGSCs (70%), endometrioid carcinomas (10%), clear cell carcinomas (10%), mucinous carcinomas (3%), and LGSC (<5%) [4]. These tumours are described as inherently diverse diseases, as indicated by differences in genetic and epidemiological risk factors; precursor lesions, pattern of spread, molecular changes in oncogenesis, response to chemotherapy and prognosis. The 10 years survival rate is estimated to be 15%–30% at later stages of ovarian cancer because of asymptomatic nature and ineffective screening tools compared to 90% survival rate for early stage [2,8,17–19].
Inspite of the current considerable progress in the management, early diagnosis is unsuccessful; the recurrence rate is increased due to resistant to chemotherapy and residual tumour. In recent times, various molecular and proliferative markers have been reported as an important prognostic factor for women with the disease along with response to various therapeutic modalities [2,3,8,18].
Determination of proliferative activity of the tumour has been reported to be of a diagnostic and prognostic value with other known clinicopathologic features in several types of cancers including those of the lymphatic system, lung, brain, breast, cervix, uterus, ovary, prostate and in soft tissue sarcoma. There are several methods which can be used to estimate the number of proliferating cells by PI. Recently Ki-67 antigen immunostaining is a promising objective for PI which needs further methodological fine tuning [6,9,10,13,20,21].
Gerdes et al., identified Ki-67 as a nuclear non-histone protein [22]. The Ki-67 expression was of great interest to identify as a marker of cell proliferation due to its complete expression in proliferating tissues and the absence in quiescent cells. The Ki-67 gene is present on the long arm of human chromosome 10 (10q25). Ki-67 is also frequently measured both as a fixed marker of proliferative activity and with multiple measurements during treatment, as a potential active intermediate or substitute marker of treatment efficacy [11,22].
MIB-1 antibody has now been established as a reference monoclonal mouse antibody for the demonstration of PI in formalin-fixed, paraffin embedded specimens for Ki-67 antigen. It reacts with the Ki-67 antigen nuclear protein which is present in two isoforms of 345 and 395 kDa. The Ki-67 antigen/MIB-1 immunostaining are present during all active phases of each cell’s cycle (G1, S, G2 and M-phase) but absent in resting cells (G0 phase- quiescent state of the cell [9,11,12,23].
Ki-67 is an easily available, more economical, rapid, used on formalin fixed paraffin embedded sections and a more reproducible biomarker available in developing countries, compared with other markers like proliferating cell nuclear antigen and bromodeoxy uridine. Ki-67 immunostaining requires only small tissue samples, allowing it to be applied even in case of patients who are candidates for Neo Adjuvant Chemotherapy (NACT) [11,12,20,24].
Ki-67 immunostaining is evaluated in the most positively stained areas and all identifiable nuclear staining is interpreted as positive immunoreactivity regardless of intensity. Immunostaining is usually confined to the nucleus and cytoplasmic positivity is observed only during mitosis. Many studies have correlated this IHC marker expression in SEOC with other prognostic markers like histologic subtype, tumour grade, FIGO stage, chemotherapy response and also with survival rates [3,6,7,9,14,15,22,23,25].
The SEOC expresses high Ki-67 LI than benign and borderline tumours [21,26,27]. Sylvia et al.,studied 60 consecutive cases of epithelial ovarian tumours and found that Ki-67 LI was highest in malignant tumours (Mean PI – 48.6±26.76) followed by borderline and lowest in benign [16]. In malignant group, serous group had a high index followed by endometroid and lowest in mucinous. They also documented that CA-125 levels did not have a significant correlation with PI. Similar results were observed in our study with a high Ki-67 LI in serous group and no correlation was found between CA125 levels with LI. Many studies have reported difference in Ki-67 expression in various histological subtype and have shown different distribution of immunostaining in serous with its subtype, mucinous, endometroid and clear cell [7,9,13,16,23]. In a retrospective study of 500 ovarian tumours by Kobel et al., Ki-67 immunohistochemical expression along with other biomarkers was assessed and it was concluded that there is marked variation in PI between different subtypes but is not of prognostic significance within any subtype [25]. In the present study also, no significant association was found between the subtypes and also serous versus nonserous histology.
Heeran MC et al., observed that Ki-67 expression increased with histopathological grade (p<0.0001) [7] and large number of authors have documented the same [10,13,18,24,26]. We also found Ki-67 LI is significantly high in tumours with a high histologic grade.
It has been documented in the serous carcinoma sub types, the Ki-67 LI was higher in the HGSC than the LGSC [2,25,28,29]. The study by Giurgea et al., noted the expression of both Ki-67 and p53 in 125 diagnosed cases of epithelial ovarian neoplasms and found serous to be the most common histological subtype (92.3%) and demonstrated high Ki-67 LI in HGSC [23]. We also observed among serous group a significant difference (p=0.001) in Ki-67 LI expression between HGSC (65.34%) as compared to the LGSC (37.96%).
The Ki-67 immunostaining pattern has been observed as focal and heterogenous in more number of low grade tumour as compared to diffuse pattern in higher grade [9,13,23]. Similar observation was noted in the present study.
The FIGO system has been identified as independent prognostic factor with higher stage reflecting more aggressive state due to change in tumour Biology [8,13]. Khouja et al., found a correlation between high Ki-67 expression with higher Grade [27], poor differentiation, ascitis, the presence of residual disease after primary surgery and advanced FIGO stage. In our study, high Ki-67 LI was noted with advanced FIGO stage (stage III-70.6) which was statistically significant (p<0.001). Munstedt K et al., found a significant correlation between Ki-67 determined tumour growth fraction and incidence of tumour recurrence (p<0.001) in early stage ovarian carcinomas [15].
Several studies which have examined the relationship between Ki-67 antigen expression and long term survival have reported that the PI is a good predictor of patient outcome in epithelial ovarian cancer. The median survival of patients whose carcinoma had a high Ki-67 expression was lower compared to patients whose tumours demonstrated low Ki-67 expression [6,7,12,30].
The assessment of the PI by Ki-67 LI determines the proliferative potential of SEOC in diagnosing the high grade, HGSC and advanced stage along with routine histopathological report as aggressive tumours. This helps in prognosis and thereby, need of tailoring chemotherapy. The high Ki-67 expression tumours have an increased chemosensitivity there by better response to chemotherapy and longer survival rate. Even patient with early stage ovarian carcinomas with high Ki-67 LI are likely to benefit from adjuvant chemotherapy despite of the tumour grade and type [3,31].
The exclusive study on clear cell adenocarcinomas indicates the survival rate of the patients with high Ki-67 antigen expression was significantly greater than for low Ki-67 antigen expression and suggested low proliferation activity may contribute to chemoresistance [32].
Conventional treatment for SEOC consists of surgical removal of tumour, followed by Platinum/Taxane based chemotherapy [33]. Currently, “sandwich therapy” is preferred for advanced stage disease of the FIGO staging system that is, NACT with interval debulking surgery and postsurgery chemotherapy [33,34].
Studies by Khandakar B et al., and Miller et al., found a decrease in Ki-67 LI subsequent to NACT and significant differences in the tumour histomorphology as compared to the indigenous neoplasms with better survival outcome [3,34].
Recent studies suggest that Ki-67 is potentially an attractive therapeutic target in cancer due to the ubiquitous expression in all proliferating cells. Inactivation of the proliferation marker Ki-67 will lead to cell death specifically in proliferating cells and thus could be a potential strategy for the treatment not only of ovarian cancer but also of numerous other malignancies [35,36].
Limitation
The limitation of the study is inability to follow up the patients and assess the prognosis, as our institute is not an oncology centre and hence, the patients are referred elsewhere.
Conclusion
SEOC have the worst prognosis among the gynecological malignant tumours. A number of factors are known to persuade survival in ovarian cancer, till date, histologic grade and FIGO stage for diagnosis are considered the most important for prognostication.
Ki-67 LI help in diagnostic and prognostication by differentiation of the morphologic types when collectively studied with architectural grade and FIGO stage, predicting the response to chemotherapy and overall survival of the patient. In recent times, it is also a potentially remarkable therapeutic target. MIB 1 antibody against Ki-67 antigen is widely available and a cost effective investigation, especially in a developing country where SEOC are on the rise. Ki-67 LI IHC marker should be included in histopathology report regularly as a diagnostic and prognostic factor there by it will pay the way for better understanding of biological behaviour and modify treatment strategies.
‡FIGO-International Federation of Gynecology and Obstetrics. **Serous carcinoma (SC), Mucinous carcinoma (MC), Clear cell carcinoma (CC), Undifferentiated carcinoma (UC) Transitional cell carcinoma (TC)
†FIGO- International Federation of Gynecology and Obstetrics.