To achieve safe haemostasis various Vascular Closure Devices (VCDs) have been developed. These systems are used routinely in the vascular interventional radiology [1]. Important properties include ease of deployment, reliable haemostasis and low complication profile.
The Femoral Introducer Sheath and Haemostasis (FISH); (Morris Innovative Inc., Indiana, USA) device belongs to the new generation of VCDs. While common VCDs like suture-mediated devices (e.g., Perclose Proglide; Abbott Vascular, IL, USA), clip-based devices (e.g., the StarClose SE; Abbott Vascular, IL, USA) or plug-based devices with collagen (e.g., AngioSeal; St. Jude Medical, MN, USA) are using non-biodegradable substances to close the arteriotomy, the FISH device is designed with a bio-absorbable patch, that promotes the remodeling of the vessel wall without synthetic materials. The FISH device achieves haemostasis by placement of a ribbon of porcine-derived Small Intestine Submucosa (SIS) attached to a cuff within the vessel wall at the arteriotomy site [2,3]. The SIS remains in place until the femoral artery is healed. The vascular plug is absorbed within 30 days. The FISH device is available in sheath sizes of 5 to 8 French.
Bavry AA et al., demonstrated the advantages of the FISH device in comparison to the manual compression [2]. In a multicenter study 297 patients underwent diagnostic coronary interventions. For the FISH device cohort the mean time to haemostasis and mean time to ambulation were reduced. There were no significant differences in the rates of complications between the FISH device and manual compression. In this study, the haemostatic outcome of the FISH device after femoral arterial access in interventional radiological procedures was reviewed.
Materials and Methods
This interventional study was performed from March 2014 to September 2015 after approval of the Institutional Review Board (IRB) in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
In 54 patients with arteriosclerotic Peripheral Artery Disease (PAD) in stage 3 to 5 according to Rutherford [4,5] a percutaneous 6F VCD was used after a vascular intervention by one experienced radiologist (with an experience of more than 5000 interventions).
Preinterventional anti-platelet therapy with oral acetylsalicylic acid (100 mg/ d) was given in 51/54 (94%) patients. 30/54 (55.6%) additional patients received dual anti-platelet therapy with aspirin and clopidrogel (75 mg/ d). Two patients (3.7%) had anticoagulation with phenprocoumon. The therapy was previously changed to subcutaneous heparin. After phenprocoumon was displaced, a prothrombin time (Quick test) of at least 50% was required. Peri-interventional, all patients received 5,000 IU heparin via the arterial access. A light compression bandage was used after realizing the closure followed by bed rest for 6 hours.
FISH device: The preparation of the closure device was carried out immediately after the angiographic intervention under sterile conditions. Prior to deployment of the closure device, dedicated angiography of the access site was performed. After intervention, the closure device was inserted over the wire. The pulsatile blood flow flash back through a side port ascertained the correct intravascular position of the device. A release wire fixed the patch on the device until it was placed in the vessel wall. The sheath was advanced into working position after removing the release wire. To activate the patch, the compression tab, located at the base of the hub, was removed and pulled until resistance was felt. While removing the sheath, the patch was plugged together into the artery wall. After release of the patch, an immediate haemostasis was estimated [Table/Fig-1a-d]. Slight oozing of blood through the access site by injured subcutaneous arterial branches, was stopped by slight compression.
External and internal view of the FISH procedure.
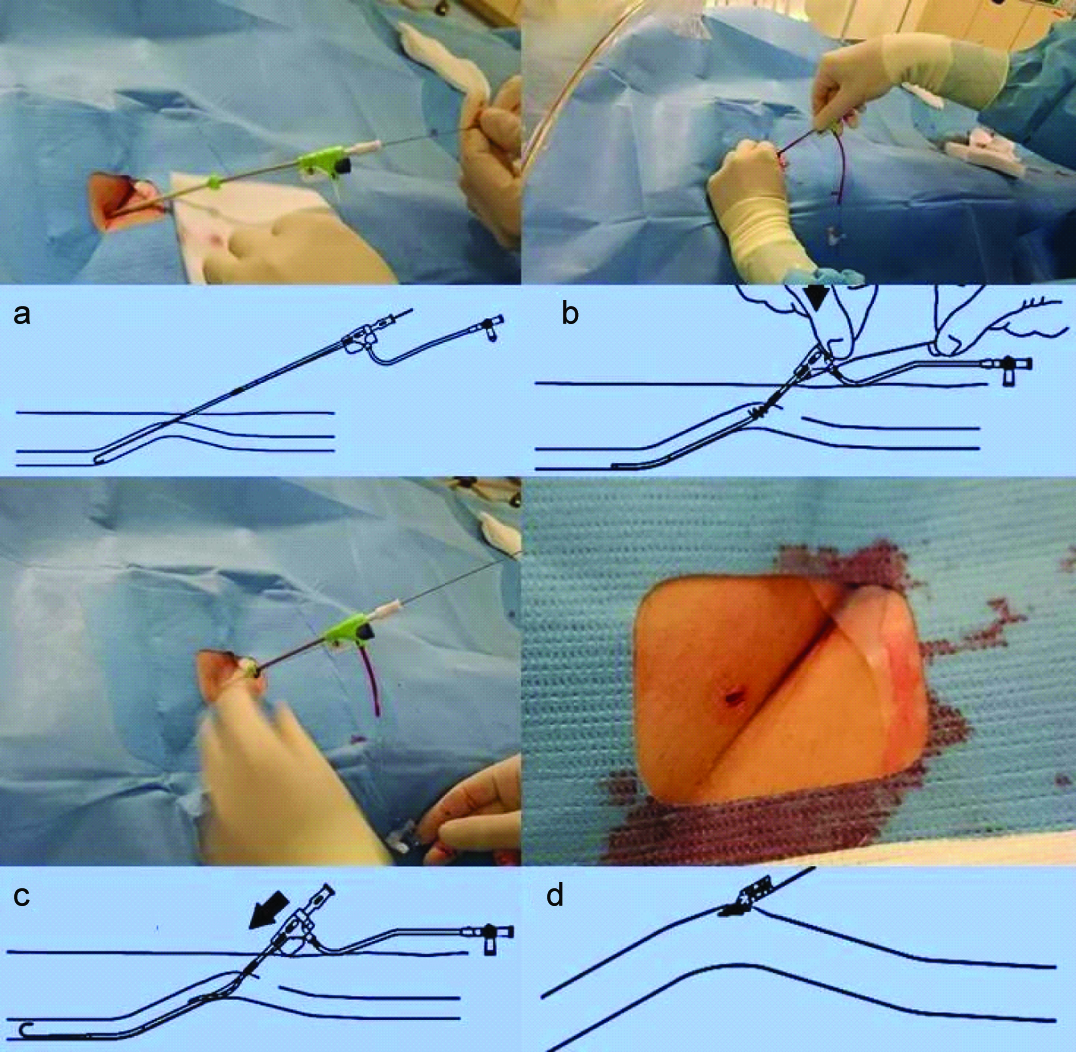
The device was not used in patients who have a known sensitivity or allergy to porcine derived material.
On the first day after the vascular intervention, all patients were examined clinically and by colour-coded ultrasound of the puncture site and the follow-up were planned after 6 weeks.
Statistical Analysis
All values are expressed as means±standard deviation or the number of patients and percent.
Results
We examined and treated 33 male and 21 female patients with an average age of 69.0±10.7 years (53-92 years). 37 (68.5%) patients had PAD stage 3, two (3.7%) patients had stage 4 and 15 (27.8%) patients had stage 5 PAD. Data for Body Mass Index (BMI) were available for all patients with an average of 25.7±3.8 (20-33). The puncture was performed in 40 (74.1%) patients in antegrade and in 14 (25.9%) patients in retrograde technique. In 50 (92.6%) patients the FISH device was applied successfully. In these cases no additional manual compression was necessary.
In 4 cases (7.4%) an immediate haemostasis was not achieved. In these cases, prolonged manual compression was utilized. In addition, a firm pressure bandage for 12 hours was applied. All 4 patients had an intervention in retrograde cross-over technique with a long 6F sheath (Terumo Destination 6F). In accordance with the Society of Interventional Radiology reporting standards for clinical evaluation of new peripheral arterial revascularization devices [5] there was one major complication in one patient (1.9%) with a retroperitoneal haemorrhage requiring transfusion. A Computer Tomography (CT) with intravenous contrast media was performed immediately after realizing the discomfort of the patient with low blood pressure (about 2 hours after intervention). The CT scan visualized a retroperitoneal haematoma without signs of an active bleeding. The patient was transferred to an intensive care unit and required blood transfusion. A surgery was not necessary. After conservative treatment this patient was free of symptoms. Minor complications like a small groin haematoma or a groin granuloma were not observed. The incomplete immediate haemostasis and the bleeding complication were related to specific conditions (i.e., cross over approach) and the result of learning curve.
Discussion
Focused on the use of the FISH device, we evaluated the safety and effectiveness in clinical routine. Depending on the mechanism of action to achieve haemostasis, the FISH device was classified into the passive approximators like the ExoSeal device (Cordis Corporation, Miami Lakes, FL, USA), while achieving haemostasis through a bioabsorbable plug in the arteriotomy site. In contrast, active approximators are designed to replicate surgical closure of an arteriotomy (e.g., suture-based or clip-based devices) [6].
In the manufacturer’s instruction for use there is a demand for training of the user by an authorized representative before using the FISH device. After a 6F approach we used the closure device regardless of the arterial access way (antegrade/ retrograde), calcifications or obesity. The mean BMI was 25.7±3.8 comparable to previous studies [3]. Overall we found a low complications rate (n= 1; 1.9%). In this patient who developed a complication, this may have been a consequence of administration of anticoagulation before, during and after the procedure and too high puncture of the Common Femoral Artery (CFA).
The advantages of this device in contrast to the common VCDs are the successful haemostasis without interfering the reparative processes of tissue injury and the complete absorption of the material leaving behind an intact vessel wall within 30 days. An immediate re-puncture is possible.
Disadvantage of the FISH device is to be careful in selecting the right FISH device size according to the sheath size. For example the external diameter of a 6 F cross-over-sheath is greater than normal short-length sheaths.
In a multi-center study of Bavry et al., the FISH-system was used successfully [2]. Thus, a primary haemostasis resulted in 187/191 patients (97.9 %). Similar to other closure systems, a primary success rate of > 90% is achievable. In our study we reached primary haemostasis in 50/54 cases (92.6 %).
In our previous study about the ExoSeal device [7], we achieved an immediate haemostasis in 939/1000 patients (93.9%) after antegrade or retrograde puncture technique. The group of Schmelter C et al., studied the ExoSeal system in antegrade vascular punctures, used it successfully in 96/100 patients (96%) [8]. Boschewitz JM et al., achieved primary haemostasis in 651/682 patients (95.5%), in repeated access [9]. In comparison to other systems such as percutaneous suture-mediated closure systems (Perclose/Proglide) show similar values. Immediate haemostasis can be achieved in 95.6% cases [10]. Overall, the usable closure systems are equivalent to each other in terms of primary haemostasis.
Malfunction of the FISH device was not observed. Malfunctions of other closure systems: for example the AngioSeal system shows relatively frequent malfunctions in 12/120 patients (10%) [11]; the Star Close- system only in 1.1% of cases (13/1213) [12]; malfunction with the ExoSeal-device were in 15/1000 (1.5%) patients [7].
Mackrell PJ et al., treated the patients with manual compression in whom the placement of the perclose suture-mediated closure system had failed [13]. The most common cause of the failure was an inability to introduce the system into the vessel, particularly in antegrade access routes together with obesity. This problem was also found in heavily calcified vessels and previous surgery. The clip-based closure system (StarClose) was used by Chiu AH et al., in 142 cases. In 11/142 (7.7%) patients the system could not be successfully placed, especially in an antegrade puncture technique [14].
In the study of Bavry AA et al., there was one death reported during the randomized investigation, which was not device related [2]. One closure method related adverse events (major complication) were seen in the clinical trial: bleeding requiring transfusion (0.7%). In our evaluation only one serious complication occurred, with haemorrhage requiring transfusion (1.9%).
The groups of Schmelter C et al., and Boschewitz JM et al., had no patients with major complication [8,9]. With a number of 8/2103 (0.4%) patient’s serious complications after the use of a percutaneous suture closure system were low [10]. They consist of deep infections and bleeding requiring transfusion. Major complications with the ExoSeal device were described very low in 1/1000 (0.1%) [7]. For the AngioSeal system a major complication rate of 0-1.1% was described [15–17]. For the Star Close- system serious complications were described in 3.5% [12,18,19].
In this study there were no minor complications observed (0%). Bavery AA et al., had 4 minor complications (2.9%): 3 groin haematomas and 1 pseudoaneurysm treated with ultrasound guided thrombin injection [2].
In the ECLIPSE study minor complications were found in 9% of the cases [19]. Minor complications after antegrade puncture technique and application of ExoSeal system were found in 7% of the cases with 4 pseudoaneurysms and 3 low rebleeding [8]. In our ExoSeal study [7] the group of minor complications consisting of groin haematomas, pseudoaneurysms and stenosis occurred in 7.4%. Boschewitz JM et al., reported a low rate of minor complications [9]. There were a total of only 8/659 patients (1.2%) with small groin haematomas. The AngioSeal system is specified with minor complication rates from 1.4 to 27.8% [15], while the recirculation clip - based system (Star Close) were detected minor complication of 5.3- 22.5% [12,14]. For the percutaneous suture-mediated system (Perclose/Proglide) pseudoaneurysms, groin haematoma and palpable groin suture-granuloma were described [10] in a total of 15.6%.
Granuloma or inflammatory reactions in the interventional access path were not found in our study. Theoretically, use of a collagen plug and suture-mediated vascular closure of an arteriotomy site can impinge on the CFA causing inflammation, remodeling and flow disturbances which potentially accelerate atherosclerosis. The process can take years to result in clinically significant artery narrowing [20].
Limitation
Limitations of this study were the retrospective consideration with typical limited values. This study focused on FISH device only. Other types of VCDs were not included in the investigation. This was a single-center study and the sample size was relatively small, e.g., for the detection of rare complications.
Conclusion
The FISH device is a safe and effective tool at access sizes of 6F, irrespective of the access technique, especially in anticoagulated patients.