Cancer is the second most common cause of death overall around the world. About 11 million new cases of cancer are diagnosed every year. Head and neck cancers account for approximately 6% of all the malignancies in the United States, and over one third of all the cancers in India [1,2]. OPMDs are a group of disorders which have a propensity to transform into oral cancer and include mainly oral leukoplakia, oral submucous fibrosis, and oral lichen planus. Interestingly, they share common aetiological factors with oral cancer, particularly the use of tobacco [3].
OSCC is multifactorial in aetiology with mutiple intricate pathways at cellular, molecular and biochemical level leading to initiation and progression of the disease. Oxidative stress is a result of an imbalance between the production of reactive species and a biological system’s ability to either counteract these products or repair the damage caused by them. The sustained inflammatory/oxidative environment leads to accumulation of genomic damage, which may lead to initiation of carcinogenic cascade [4].
Nitric oxide free radicals (NO•), one of the free radical implicated in carcinogenesis, is a short lived free radical generated from l-arginine by the enzyme Nitric Oxide Synthase (NOS) [4] [Table/Fig-1]. The toxicity of nitric oxide is linked to its ability to combine with superoxide anions to form peroxynitrite, an oxidising free radical that can cause DNA fragmentation and lipid peroxidation [5]. Nitric oxide modulates different cancer-related events including angiogenesis, apoptosis, cell cycle, invasion, and metastasis. However, nitric oxide has been shown to have dual effect in carcinogenesis where it has also been reported to have tumouricidal effects [6]. Enzymatic antioxidants (e.g., Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), glutathione reductase, catalase) and nonenzymatic antioxidants (e.g., Glutathione (GSH), vitamins C and D) are groups of molecules which act as antagonist to the oxidant species within the body [4]. Vitamin C also known as ascorbate or Ascorbic Acid (AA) are basically a ketolactone that has reported molecular weight of 176.13 g/ml and is considered to be one of the strongest reducing agents as well as a radical scavenger. Ascorbate can reduce nitrates and prevents the formation of carcinogenic nitrosamines. The cytotoxicity induced by ascorbate seems to be primarily mediated by accumulation of hydrogen peroxide [Table/Fig-2] [7]. Estimation of antioxidant and oxidant levels by calculating AOI in OPMD and OSCC could serve as an objective biomarker for monitoring disease progression. Thus, the present study was designed to assess the AOI through estimation of serum levels of nitric oxide and vitamin C in OPMD and OSCC patients.
Materials and Methods
This cross-sectional study was carried out in ITS Dental College, Ghaziabad, Uttar Pradesh, India, and comprised of 50 subjects who were randomly selected using purposive sampling, which included 10 cases of healthy controls, 20 cases each with histopathologically proven diagnosis of oral potentially malignant disorders (oral leukoplakia, oral submucous fibrosis and oral lichen planus) and OSCC each. A written informed consent was obtained from all the subjects. Inclusion criteria for the study group consisted of patients with definitive clinical and histopathological diagnosis of oral submucous fibrosis, oral leukoplakia, oral lichen planus and primary OSCC. Exclusion criteria was past history of any major illness such as liver disease, tuberculosis, diabetes and hypertension or with any history of malignancy other than oral cancer; recurrent or secondary lesions; patients undergoing radiotherapy or chemotherapy and subjects who were on antioxidants/multivitamin preparations were excluded. The healthy controls had no habit of tobacco, alcohol, were devoid of any chronic illness and were not on any long term medication.
Venous blood was collected following aseptic precautions and the serum was separated by centrifuge machine at 3000 rpm for 10 minutes and utilized for biochemical analysis. The serum levels of vitamin C were estimated by the phenyl-hydrazine spectrophotometry method as done by Lowry OH et al., and serum nitric oxide levels were estimated using the Greiss reaction where Greiss reagent reacts with nitric oxide to form a purple-coloured complex whose absorbance is read at 546 nm as reported by Green LC et al., [8,9] Oxidant- antioxidant ratio indicative of oxidative stress was calculated by taking the ratio of concentration of nitric oxide with concentration of vitamin C in serum of study groups. AOI was calculated by calculating the ratio between serum levels of nitric oxide and vitamin C in all study samples.
Statistical Analysis
The data was recorded and analyzed statistically using SPSS software version 20.0 using ANOVA and post-hoc Bonferroni tests. The difference was considered statistically significant when p-value were 0.05 or less.
Results
The study comprised of 50 subjects, out of which 43 were males (controls-5; OPMD-18; OSCC-20) and seven were females (controls-5; OPMD-2). The mean age was 26.4±1.58 years in control group, 39.30±10.57 years in OPMDs and 49.25±13.58 years in OSCC. Out of 20 OPMD patients, two (10%) were without any habit, nine (45%) with tobacco chewing, three (15%) with smoking and six (30%) with both tobacco chewing and smoking. Among 20 OSCC patients, one (5%) was without any habit, six (30%) with habit of tobacco chewing, four (20%) with smoking and nine (45%) with both smoking and tobacco chewing. Among OPMD patients, in 15 cases, the lesion was present on the buccal mucosa, three cases were present on labial mucosa, in n=1 (5%) case was present on both labial and buccal mucosa and n=1 (5%) was present on the alveolus. Among n=20 OSCC patients, in n=7 (35%) cases, lesions were present on buccal mucosa, in n=6 (30%) patients was present on tongue, in n=3 (15%) cases was present on tonsil, n=2 (10%) was present on alveolus, n=1 (5%) was present on palate and n=1 (5%) was present on retromolar trigone.
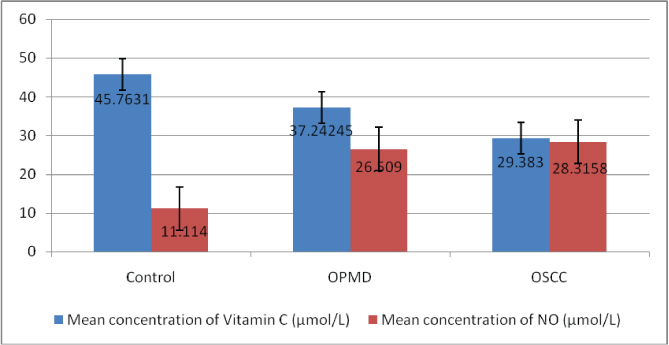
The mean nitric oxide level was 28.31±5.57 μmol/l in OSCC group, 26.5±2.46 μmol/l in OPMD group and 11.11±0.99 μmol/l in control group. The mean serum vitamin C level was 29.38±4.08 μmol/l in OSCC group whereas, it was 37.24±8.61 μmol/l in OPMDs group and 45.76±2.58μmol/l in control group [Table/Fig-3]. The difference in levels of nitric oxide and vitamin C were statistically significant (ANOVA, p<0.05). A significant correlation in the levels of serum nitric oxide when comparing between control and OPMD and control and OSCC (p<0.05) but difference in nitric oxide levels were not significant between OPMD and OSCC group (p>0.05). Levels of vitamin C show significant correlation between all the groups, the carcinoma and OPMD group, and carcinoma and control group as well as between OPMDs and control group [Table/Fig-4]. There was significant increase in AOI from control group (0.023), OPMDs (0.167) and OSCC group (0.279) (p<0.05) [Table/Fig-5,6].
Comparison of mean serum nitric oxide and vitamin C levels in control, OPMD and OSCC groups.
Pairwise comparison of serum nitric oxide and vitamin C levels in control and study groups.
| Variables | (I) Group | (J) Group | MeanDifference(I-J) | Std. Error | p*-value |
|---|
| VitaminC | Control | OPMD | 8.521 | 2.389 | 0.003 |
| Control | OSCC | 16.380 | 2.389 | 0.000 |
| OPMD | OSCC | 7.859 | 1.951 | 0.001 |
| NitricOxide | Control | OPMD | -15.395 | 1.510 | 0.000 |
| Control | OSCC | -17.201 | 1.510 | 0.000 |
| OPMD | OSCC | -1.807 | 1.233 | 0.449 |
*Post-hoc Bonferroni’s analysis with ANOVA, p-value <0.05 considered statistically significant
AOI in controls and study groups.
| Variable | Group | N | Mean | Std. Deviation | p-value |
|---|
| AOI | Control | 10 | 0.243 | 0.023 | <0.001 |
| OPMD | 20 | 0.741 | 0.166 |
| Cancer | 20 | 0.988 | 0.279 |
*ANOVA, p-value <0.05 considered statistically significant
Pairwise comparison of AOI index in control and study groups.
| DependentVariable | (I) Group | (J) Group | MeanDifference(I-J) | p-value |
|---|
| AOI | Control | OPMD | -0.49832* | 0.001 |
| Cancer | -0.74556* | 0.001 |
| OPMD | Control | 0.49832* | 0.001 |
| Cancer | -0.24723* | 0.001 |
| Cancer | Control | 0.74556* | 0.001 |
*Post-hoc Bonferroni’s analysis with ANOVA, p-value <0.05 considered statistically significant
Discussion
Oral carcinogenesisis follows a complex, multistep course where a series of complex cellular, molecular and biochemical changes occur in response to varying endogenous and exogenous stimuli. One of the significant pathways involves generation of free radicals in the tumour microenvironment [10].
Free radicals are unstable and highly reactive due to odd number of electron. They have the property of gaining stability by taking an electron from another stable electron, eventually making that molecule unstable. The attacked molecule loses its electron becoming a free radical itself initiating a cascade of free radical formation causing alteration in the cells [10].
Under pathological conditions, much larger amounts of free radicals are formed than normal. To mitigate their harmful effects, antioxidant defence mechanisms act at different levels. ‘Antioxidant’’ refers to any molecule capable of stabilizing or deactivating free radicals before they attack cells. Weakened antioxidative defence mechanisms result in oxidative-antioxidant imbalance. Reduced activities of antioxidants with concomitant increased levels of oxidative stress have been reported in different cancers including head and neck [10].
The known classic risk factor of oral cancer is tobacco use. Tobacco products contain a number of carcinogens, such as benzopyrene and other polycyclic aromatic carcinogens which are the most important carcinogenic agents in cigarette smoke, whereas, nitrosamines are the strongest carcinogens in smokeless tobacco [11]. Tobacco increases production of free radicals such as nitric oxide and superoxide anions and yield by-products which are capable of inducing various type of stress and biological effects involved in the process of carcinogenesis [11].
In our study, levels of nitric oxide were lowest in control group but increased significantly in OPMDs and OSCC groups. The levels of nitric oxide in OPMDs and OSCC were comparable. This is in accordance with the study done by Patel JB et al., who also reported increased level of nitric oxide in OPMD and OSCC patients [12]. Yang L et al., and Augustine D et al., reported increased level of inducible nitric oxide synthetase enzyme in the pathological grades of OSCC and also found it to be positively correlated with the expression of p53 [13,14]. In a investigation [11] the expression of iNOS in dysplasia was studied and linked with p53 expression, it was observed that the Inducible NOS (iNOS) level augmented the severity of the dysplasia and linked with p53 expressions, based on these observations it was concluded that there was a likelihood that this association can be a significant contributor in development of malignancy from premalignant as well as in-situ lesions. However, studies with divergent results have shown expression of nitric oxide to be associated with tumouricidal activities [11]. The significant rise in concentration in OPMDs and OSCC cases indicates persistent oxidative stress compared to normal subjects which may activate proto-oncogenes and transcription factors, genomic instability, chemotherapy-resistance, invasion and metastasis.
Serum levels of vitamin C were highest in control group and reduced significantly in OPMDs and OSCC group in the present study. There was statistically significant difference in the levels of vitamin C in OPMDs and OSCC also. Reduced level of vitamin C in OPMDs and OSCC compared to healthy individuals in our study represent the drained antioxidant scavenger action of the body due to increased oxidative stress as depicted by increased nitric oxide levels also. This is in accordance with the study done by Vashistha A et al., who reported reduced level of vitamin C in OSMF and OSCC [15], Basu S who found out reduced level of vitamin C in leukoplakia [3], OSMF and OSCC and with Guruprasad R et al., who also revealed reduced level of vitamin C in OSMF [16].
De Munter L et al., and Edefonti V et al., reported a strong inverse correlation between vitamin C intake and risk of head and neck cancer [17,18]. Also, Venturelli S et al., reported that anticancer effect of vitamin C is dose dependent. In high doses, it acts as a pro oxidative drug, and its effect is mediated by reactive oxygen species and ascorbyl radicals. It catalyses the hydrogen peroxide production in the cancer tissue and therefore high dose ascorbate has been used in complementary medicine [19]. However, in a randomized clinical trial Nagao T et al., assessed the use of vitamin C and beta carotene in the treatment of oral leukoplakia and found that vitamin C was ineffective for clinical remission or in prevention of development of cancer [20].
Ascorbic acid is a potent antioxidant or reducing agent that is capable of scavenging free radicals of reactive oxygen and nitrogen species (ROS and RNS) that have potential to damage nucleic acids and promote carcinogenesis. The ascorbate reacts with ROS, quenches them and gets converted into poorly reactive semi hydroascorbate radical. Therefore, ascorbate efficiently decreases in vivo damage to proto oncogenes and tumour suppressor genes, countering the oxidative stress induced by reactive free radicals [21].
Few contradictory studies regarding the effect of vitamin C and its effect on cancer cells have been reported in literature. Vitamin C has been documented to augment chemical carcinogenesis in a rodent model, possibly due to its pro-oxidant activity and the subsequent increase in the formation of free radical by the chemical carcinogen 7,12-dimethylbenz[a]anthracene [22–24]. Some literature also states that AA when applied in low concentrations has been found to be an indispensable prerequisite for the development of murine myeloma cells in cell culture model. However, few others have reported that vitamin C inhibits the development of cancer cells at greater concentrations in a dose dependent manner [7,25,26].
AOI calculated by taking the ratio between concentration of nitric oxide and vitamin C is a more sensitive assay for assessment of oxidative stress in the lesional microenvironment. To our knowledge, this is the first study comparing ratio of serum levels of antioxidants to oxidants providing a comprehensive index to monitor disease progression in OPMDs and OSCC patients. AOI offers a more objective assessment of oxidative status of the disease. AOI provides a comprehensive assessment of proportional levels of antioxidant and oxidants in the body. Progressive increase in the AOI may provide a pro-oxidant environment for the progression of the lesion from potentially malignant disorder to oral cancer. Regular monitoring of AOI over regular intervals with clinicopathologic correlation can be a useful biomarker for understanding oral cancer evolution.
Limitation
The limitations of the study include relatively smaller sample size and lack of prospective follow up of the cases due to cross-sectional design of the study. Studies with larger sample size and regular follow up of the patients should be undertaken to ascertain AOI as an objective tool which could prove to be of therapeutic significance. The results of the present study need to be established with multicentric, large population based studies to validate the role of AOI as a simple, minimally invasive preventive biomarker for oral cancer.
Conclusion
Oral carcinogenesis follows a complex multistep progression model with an array of genetic, epigenetic, phenotypic and biochemical changes occurring at each level. Imbalance at oxidant-antioxidant level may result in accumulation of toxic free radicals with deficiency of radical scavengers within the cell. The results of the present study elucidate the role of oxidant-antioxidant levels and AOI as an easy, minimally invasive and sensitive biochemical assay to monitor development of oral carcinogenesis from precancer to cancer.
*Post-hoc Bonferroni’s analysis with ANOVA, p-value <0.05 considered statistically significant
*ANOVA, p-value <0.05 considered statistically significant
*Post-hoc Bonferroni’s analysis with ANOVA, p-value <0.05 considered statistically significant