Introduction
Vanillic acid, a naturally occurring bioactive substance, possesses diverse pharmacological potential including free radical scavenging and anticancer properties. Excessive generation of Reactive Oxygen Species (ROS) and insufficient antioxidant potential has been involved in numerous pathological disorders, including cancer.
Aim
To explore the anti-lipid peroxidative and antioxidant efficacy of vanillic acid in Dimethylbenz[a]Anthracene (DMBA) induced oral carcinogenesis.
Materials and Methods
Topical application of DMBA for 14 weeks in the buccal pouch of hamsters resulted in well developed oral squamous cell carcinoma. Vanillic acid at a dosage of 200 mg/kg body weight was orally administrated to the hamsters for 14 weeks. The status of lipid peroxidation and antioxidants were measured in the plasma and buccal mucosa of hamsters using specific colorimetric methods.
Results
Altered levels of lipid peroxidation by-products {Thiobarbituric Acid Reactive Substances (TBARS)} and disturbances in antioxidants status {Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPx), vitamin E, vitamin C and reduced Glutathione (GSH)} were observed in the plasma and buccal mucosa tissues of hamsters treated with DMBA alone. Vanillic acid (200 mg/kg bw p.o) significantly restored the above mentioned plasma and buccal mucosa biochemical variables to near normal range in DMBA treated hamsters.
Conclusion
Present findings thus confirm the anti-lipid peroxidative and antioxidant efficacy of vanillic acid in DMBA induced hamster buccal pouch carcinogenesis.
Catalase, Glutathione peroxidase, Oral carcinoma, Oxidative stress, Reactive oxygen species
Introduction
Oral cancer is the widespread malignant neoplasm and the fifth most common cancer globally [1]. In spite of recent progression in the cancer treatment, the morbidity as well as mortality due to oral cancer is still greater in developing countries including India. Oral cancer accountability is 40-50% of all cancers. The prime risk factor of oral cancer is tobacco and alcohol consumption, and 80% of oral cancer patients are accustomed to tobacco and alcohol utilization [2]. Out of all oral cancer cases, 90% of the cases are squamous cell carcinoma and its five year survival rate is still poor [3].
Oral cancer develops through three stages, initiation, promotion and progression. Golden Syrian hamsters are the ideal model to understand the pathogenesis of human oral cancer [4]. 7,12- DMBA, an effective site specific carcinogenic agent, is generally used to induce cancer in oral (buccal pouch), skin and mammary tissues [5]. DMBA induces cancer through alteration of DNA base pairs, initiation of chronic inflammation and excessive generation of Reactive Oxygen Species (ROS). Cancerous and precancerous lesions of DMBA induced tumours of hamster buccal mucosa closely resemble the human oral squamous cell carcinoma. Golden Syrian hamsters are generally used and commonly accepted model for exploring the chemopreventive potential of natural entities and synthetic drugs [6].
Lipid peroxidation, a chain reaction mediated by free radicals, oxidizes the lipids, predominantly Polyunsaturated Fatty Acids (PUFA). During the cell division, lipid peroxides play a key role to control the cell division. Profound studies reported an inverse correlation between lipid peroxidation and cell proliferation [7]. Previous studies explored low levels of TBARS in the tumours of the oral squamous cell carcinoma [8]. Lipid peroxidation has been implicated by over-production of ROS, causing altered structural integrity of the cell membrane, DNA, proteins and lipids. Living organisms have different antioxidant systems that counteract the adverse effect of ROS. Over-generation of ROS and poor activities of antioxidant defense mechanism results in oxidative stress, which in turn is involved in numerous pathological disorders, including cancer [9].
The liver detoxifies and counteracts the mutagenic and carcinogenic substances [10]. Glutathione-S-Transferase (GST), Glutathione Reductase (GR) and reduced GSH detoxify the carcinogen by depleting their reactive centres or facilitating their excretion by conjugation process [11]. Assessment of phase I and phase II detoxification enzymes in the liver and buccal mucosa could help to assess the chemopreventive potential of the test entities.
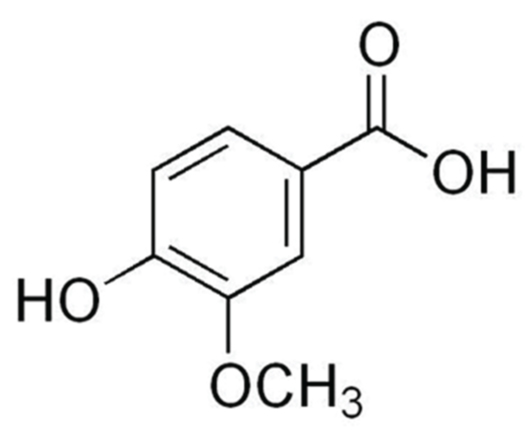
Angelica sinensis has several phytochemicals and is an important source of natural antioxidants. Vanillic acid [Table/Fig-1] is a dietary phenolic compound that protects biological membrane and inhibits lipid peroxidation in cells [12]. Profound studies reported that vanillic acid has the potential to scavenge or eliminate the ROS including hydroxyl radicals and lipid peroxy radicals [13]. Vanillic acid also exerted antimicrobial [14], anti-inflammatory [15], anti-tumourigenic [16] and hepatoprotective effects [17]. The present study was focused to assess the anti-lipid peroxidative and antioxidant potential of vanillic acid in 7,12 DMBA induced hamster buccal pouch carcinogenesis.
Molecular structure of vanillic acid.
Materials and Methods
The present study was an in vivo study conducted in the Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, Annamalainagar, Tamil Nadu, India. The total study period was 16 weeks (four months), The local institutional animal ethics committee (Registration Number 160/1999/CPCSEA), Annamalai University, Annamalainagar, India, approved the experimental design (Proposal No.1106, dated 09.10.2014, sample size 24 hamsters).
DMBA and vanillic acid were purchased from Sigma-Aldrich Chemicals Pvt. Ltd., Bengaluru, India. All other chemicals used were of analytical grade purchased from Himedia laboratories, Bengaluru, India.
Experimental Animal Model Used
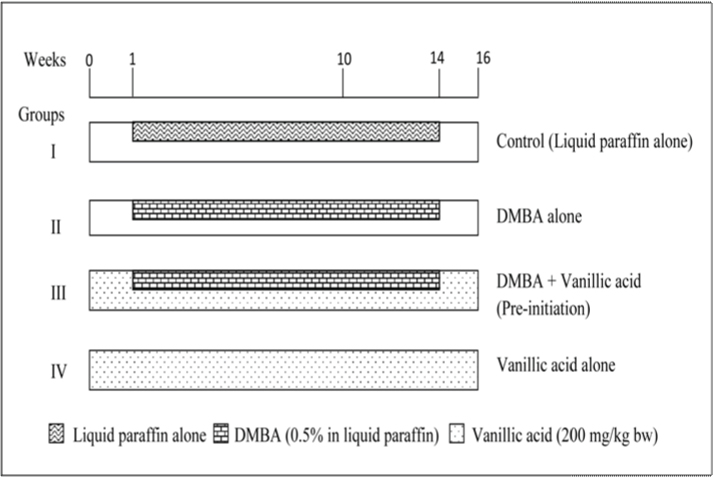
The animals (golden Syrian hamsters-male, 56-70-day-old, weighing 80-120g) were purchased from National Institute of Nutrition (NIN), Hyderabad, India and maintained at Central Animal House, Annamalai University, Annamalainagar, India. The hamsters were housed in controlled environment. The hamsters were maintained according to the principles and guidelines of the ethical committee for animal care of Annamalai University. Oral squamous cell carcinomas were induced in each hamster’s buccal pouch (left side only) with topical application of DMBA (0.5%) in liquid paraffin three times a week for 14 weeks.
Experimental Protocol
A total of 24 hamsters were divided into four groups of six hamsters in each. The experimental protocol followed in the present study is given in [Table/Fig-2].
Biochemical Estimations
Blood samples (3 ml) were collected into heparinized tubes. Plasma was separated by centrifugation at 1000 rpm for 15 minutes. Tissue samples from hamsters were washed with ice cold saline and dried between folds of filter paper, weighed and homogenized using appropriate buffer (TBARS: 0.025 M Tris-HCL buffer, pH 7.5; SOD: 0.025 M sodium pyrophosphate buffer pH 8.3; CAT: 0.01 M phosphate buffer, pH 7.0; GSH and GPX: 0.4 M phosphate buffer, pH 7.0) in an all glass homogenizer with Teflon pestle and used for biochemical estimations.
TBARS were assayed in the plasma and buccal mucosa according to the methods of Yagi K and Ohkawa H et al., respectively [18,19]. The levels of enzymatic antioxidants such as SOD, CAT and GPx were estimated by the methods of Kakkar P et al., Sinha AK and Rotruck JT et al., respectively [20–22]. Reduced GSH was determined by the method of Beutler E and Kelley BM [23]. Vitamin C and E were measured by methods of Omaye ST et al., and Desai ID respectively [24,25].
Statistical Analysis
All quantitative data are expressed as mean±Standard Deviation (SD). The statistical comparisons were performed by one-way analysis of variance (ANOVA), followed by DMRT. The results were considered statistically significant if the p-values were less than 0.05. The data were analyzed using SPSS 17.0 package.
Results
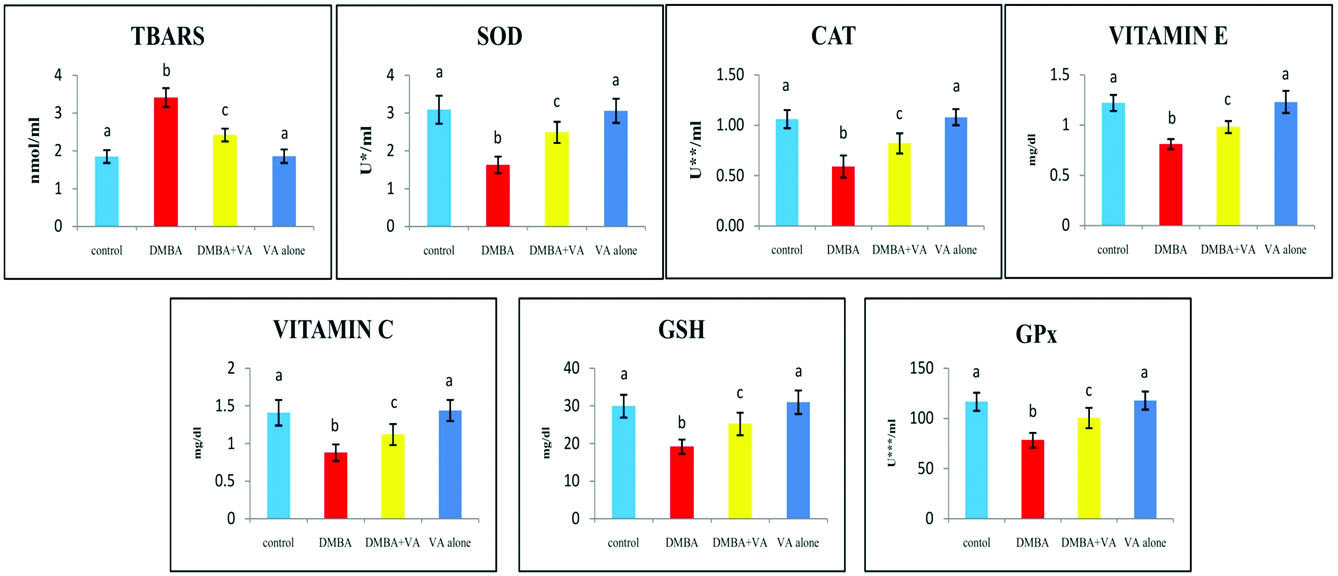
The status of TBARS and antioxidants (SOD, CAT, GPx, vitamin E, vitamin C and GSH) in the plasma of control and experimental hamsters in each group are shown in [Table/Fig-3]. The concentration of TBARS was raised, whereas the status of the antioxidants was substantially decreased in the DMBA alone treated hamsters, as compared to control hamsters. DMBA+vanillic acid treated hamsters showed near normal levels of TBARS and antioxidants.
The status of plasma TBARS and antioxidants in control and experimental hamsters in each group (n=6).
Values are expressed as mean±standard deviation for six hamsters in each group. Values that do not share a common superscript between two groups differ significantly at p < 0.05 (DMRT). * - the amount of enzyme required to inhibit 50% NBT reduction; ** - micromoles of hydrogen peroxide utilized/s; *** - micromoles of glutathione utilized/min
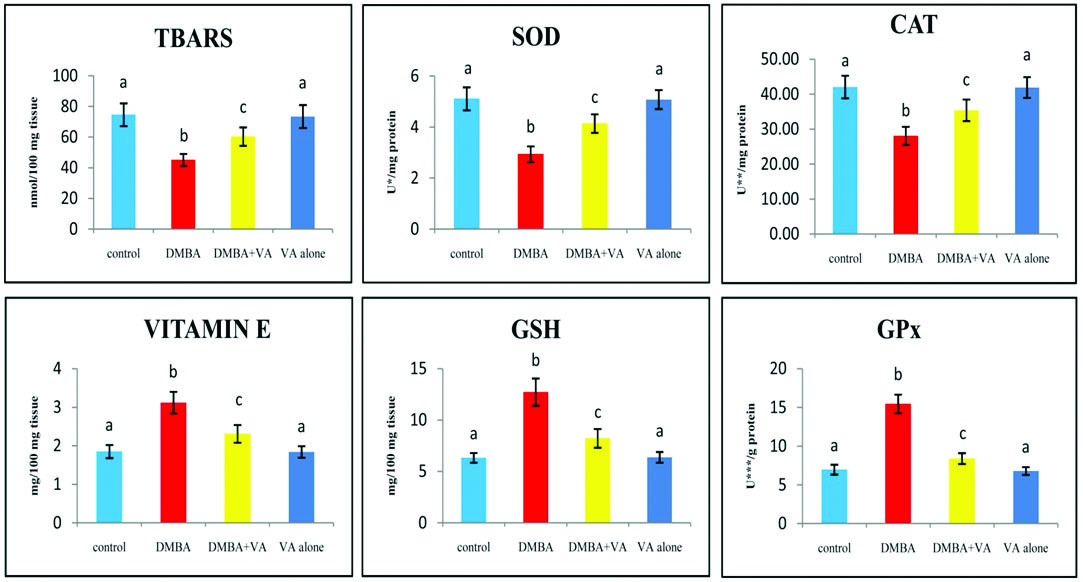
The levels of TBARS and antioxidants (SOD, CAT, vitamin E, GSH and GPx) in the buccal mucosa of control and experimental hamsters in each group are shown in [Table/Fig-4]. TBARS, SOD and CAT levels were decreased and vitamin E, GSH and GPx levels were increased in tumour bearing hamsters as compared with control hamsters. Vanillic acid alone treated experimental animals showed no significant difference in TBARS and antioxidants levels, as compared to control hamsters. Vanillic acid administration to the DMBA treated hamsters brought back the status of buccal mucosa TBARS and antioxidants to the near normal range.
The status of buccal mucosa TBARS and antioxidants in control and experimental hamsters in each group (n=6).
Values are expressed as mean±standard deviation (S.D.) for six hamsters in each group. Values that do not share a common superscript between two groups differ significantly at p<0.05 (DMRT). * - the amount of enzyme required to inhibit 50% NBT reduction; ** - micromoles of hydrogen peroxide utilized/s; *** - micromoles of glutathione utilized/min
Discussion
In this study, DMBA alone treated hamsters showed higher levels of TBARS in the plasma and lower levels of TBARS in the buccal mucosa tissues as compared with control hamsters. A number of research reports have established the potential of DMBA and their by-products to stimulate the creation of ROS such as peroxides and superoxide anion radicals, which causes oxidative stress in the cells [26]. Tissue damage may be evaluated by assessing the lipid peroxidation products and antioxidant status [27]. Lipid peroxidation by-products that leak from the site of inflammation enter into circulation to provoke damage at distinct tissues. Elevated plasma lipid peroxidation by-products have been well documented in oral carcinogenesis [28].
Vitamin C is a vital water soluble vitamin and potent reducing agent in humans and other animals. Vitamin C scavenges the oxygen anion, hydrogen peroxide, hydroxyl radicals and singlet oxygen. It plays a key role in the resuscitation of α-tocopherol and defenses the plasma lipids against lipid peroxidation [29]. Vitamin E is the initial defence mechanism to protect the peroxidation of biomembrane polyunsaturated fatty acids. Profound studies explored an inverse association between lipid peroxidation and vitamin E status in oral cancer [30]. GSH plays multiple biological activities including the prevention of oxidative DNA damage, scavenging of hydroperoxides and in the protection of the biological membrane. Chen KH and Chang ML reported that vitamin C plays a vital role in the regeneration of plasma vitamin E [31]. Vitamin E improves the ascorbate and glutathione levels in the plasma [32]. Vitamin E and vitamin C play a crucial role in the maintenance of glutathione by preventing its oxidation. Insufficient antioxidant status could account for enhanced plasma lipid peroxidation. Lowered TBARS in tumour tissues might be due to low availability of PUFA or due to disturbed antioxidant status.
Enzymatic and non-enzymatic antioxidants scavenge the free radicals, decrease oxidative stress and lessen the adverse effect of ROS. Superoxide dismutase and catalase activities were lowered in the tumour tissues of DMBA treated animals. The present results indicate that the lowered activities of these enzymes could be due to accumulation of superoxides and hydrogen peroxides. Decreased activities of superoxide and catalase have been reported in a spectrum of tumour tissues [33]. The present results are in agreement with these findings.
Glutathione peroxidases are important enzymes that eliminate hydrogen peroxides in the cytosol and mitochondria by converting reduced GSH into oxidized glutathione (GSSH). Glutathione and glutathione peroxidase have been reported to have a regulatory effect on cell proliferation [34]. Increase in glutathione peroxidase and reduced glutathione could account for lowered oxidative stress [35]. DMBA is the preferred site specific chemical carcinogen to induce several types of cancers in experimental animals, including oral, skin and mammary cancers. DMBA elicits its carcinogenic response via excessive generation of ROS, induction of chronic inflammation and excessive oxidative DNA damage [36,37].
In the present study, vanillic acid administration at a dose of 200 mg/kg bw improved the status of lipid peroxidation and antioxidants in both plasma and buccal mucosa tissues of tumour bearing hamsters, which clearly indicates its potent antioxidant efficacy during DMBA induced hamster buccal pouch carcinogenesis. The present study thus concludes that the antioxidant efficacy of vanillic acid could be partly responsible for its antitumour potential during DMBA induced oral carcinogenesis.
Limitation
The present study observed that hamsters treated with DMBA+vanillic acid revealed hyperplasia, hyperkeratosis and dysplastic changes in the buccal mucosa, which suggests that vanillic acid delayed the tumour formation rather than inhibition of tumour formation. Further studies are therefore warranted to extend the duration of the experimental design to confirm the anticancer potential of vanillic acid during DMBA induced hamster buccal pouch carcinogenesis.
Conclusion
The present study, for the first time, revealed the anticancer potential of vanillic acid in DMBA induced oral carcinogenesis by utilizing the status of lipid peroxidation and antioxidants as biochemical end points.
[1]. Akram S, Mirza T, Aamir Mirza M, Qureshi M, Emerging Patterns in Clinico-pathological spectrum of oral cancersPak J Med Sci 2003 29(3):783-87. [Google Scholar]
[2]. Rodu B, Jansson C, Smokeless tobacco and oral cancer: A review of the risks and determinantsCrit Rev Oral Biol Med 2004 15(5):252-63. [Google Scholar]
[3]. Feller L, Lemmer J, Oral squamous cell carcinoma: Epidemiology, clinical presentation and treatmentJ Can Ther 2012 3(1):263-68. [Google Scholar]
[4]. Vairaktaris E, Spyridonidou S, Papakosta V, Vylliotis A, Lazaris A, Perrea D, The hamster model of sequential oral oncogenesisOral Oncol 2008 44(4):315-24. [Google Scholar]
[5]. Alias LM, Manoharan S, Vellaichamy L, Balakrishnan S, Ramachandran CR, Protective effect of ferulic acid on 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino miceExp Toxicol Pathol 2009 61(3):205-14. [Google Scholar]
[6]. Rajasekaran D, Kowsalya R, Selvasundaram R, Manoharan S, Enicostemma littorale protects cell surface abnormalities during DMBA-induced hamster buccal pouch carcinogenesisInt J Pharm Bio Sci 2015 6(1b):104-09. [Google Scholar]
[7]. Ray G, Husain SA, Oxidants, antioxidants and carcinogenesisInd J Exp Biol 2002 40(11):1213-32. [Google Scholar]
[8]. Kolanjiappan K, Ramachandran CR, Manoharan S, Biochemical changes in tumour tissues of oral cancer patientsClin Biochem 2003 36(1):61-65. [Google Scholar]
[9]. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J, Free radicals and antioxidants in normal physiological functions and human diseaseInt J Biochem Cell Biol 2007 39(1):44-84. [Google Scholar]
[10]. Sheweita SA, Drug-metabolizing enzymes mechanisms and functionsCur Drug Metab 2000 1(2):107-32. [Google Scholar]
[11]. Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL, Glutathione dysregulation and the etiology and progression of human diseasesBiol Chem 2009 390(3):191-214. [Google Scholar]
[12]. Ramachandran V, Deepa Mol SJ, Raja B, Combined effects of vanillic and syringic acids on hepatic markers, lipid peroxides and antioxidants in acetaminophen induced hepatotoxicity in wistar rats: Biochemical and histopathological evidencesPharmacologyonline 2010 2:475-86. [Google Scholar]
[13]. Prince PSM, Rajakumar S, Dhanasekar K, Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic ratsEur J Pharmacol 2011 668(1-2):233-40. [Google Scholar]
[14]. Delaquis P, Stanich K, Toivonen P, Effect of pH on the inhibition of Listeria Spp. by vanillin and vanillic acidJ Food Prot 2005 68(7):1472-76. [Google Scholar]
[15]. Kim MC, Kim SJ, Kim DS, Jeon YD, Park SJ, Lee HS, Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophagesImmuno pharmacol Immunotoxicol 2011 33(3):525-32. [Google Scholar]
[16]. Guimaraes CM, Giao MS, Martinez SS, Pintado AI, Pintado ME, Bento LS, Antioxidant activity of sugar molasses, including protective effect against DNA oxidative damageJ Food Sci 2007 72(1):C039-43. [Google Scholar]
[17]. Singh M, Tiwari V, Jain A, Ghoshal S, Protective activity of picroliv on hepatic amoebiasis associated with carbon tetrachloride toxicityIndian J Med Res 2005 121(5):676-82. [Google Scholar]
[18]. Yagi K, Lipid peroxides and human diseasesChem Phys Lipids 1987 45(2-4):337-51. [Google Scholar]
[19]. Ohkawa H, Ohishi N, Yagi K, Assay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem 1979 95(2):351-58. [Google Scholar]
[20]. Kakkar P, Das B, Viswanathan PN, A modified spectrophotometric assay of superoxide dismutaseInd J Biochem Biophys 1984 21(2):130-32. [Google Scholar]
[21]. Sinha AK, Colorimetric assay of catalaseAnal Biochem 1972 47(2):389-94. [Google Scholar]
[22]. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG, Selenium: Biochemical role as a component of glutathione peroxidaseScience 1973 179(4073):588-90. [Google Scholar]
[23]. Beutler E, Kelly BM, The effect of sodium nitrite on red cell GSHExperientia 1963 19:96-97. [Google Scholar]
[24]. Omaye ST, Turnbull TD, Sauberlich HE, Selected method for the determination of ascorbic acid in animal cells, tissues and fluidsMethods Enzymol 1979 62:311 [Google Scholar]
[25]. Desai ID, Vitamin E analysis methods for animal tissuesMethods Enzymol 1984 105:138-47. [Google Scholar]
[26]. Batcioglu K, Karagözler AA, Ozturk IC, Genc M, Bay A, Ozturk F, Comparison of chemopreventive effects of Vitamin E plus selenium versus melatonin in 7,12-dimethylbenz(a)anthracene-induced mouse brain damageCancer Detect Prev 2005 29(1):54-58. [Google Scholar]
[27]. Gutteridge JMC, Lipid peroxidation and antioxidant as biomarkers of tissues damageClin Chem 1995 41(12):1819-28. [Google Scholar]
[28]. Manoharan S, Kowsalya R, Silvan S, Baskaran N, Anusuya C, Anti-lipid peroxidative potential of glycyrrhetinic acid in 7, 12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesisInt J Res Pharm Sci 2011 2(4):596-600. [Google Scholar]
[29]. Rahman K, Studies on free radicals, antioxidants, and co-factorsClin Interv Aging 2007 2(2):219-36. [Google Scholar]
[30]. Manoharan S, Wani SA, Vasudevan K, Manimaran A, Prabhakar MM, Karthikeyan S, Saffron reduction of 7, 12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesisAsian Pac J Cancer Prev 2013 14(2):951-57. [Google Scholar]
[31]. Chen KH, Chang ML, Effect of dietary vitamin E and vitamin C on respiration and swelling of guinea pig mitochondriaJ Nutr 1978 108(10):1616-20. [Google Scholar]
[32]. Ismail NM, Harun A, Yusof AA, Zaiton Z, Marzuki A, Role of Vitamin E on Oxidative Stress in SmokersMalays J Med Sci 2002 9(2):34-42. [Google Scholar]
[33]. Ibrahim W, Lee US, Yen HC, Clair DKS, Chow CK, Antioxidant and oxidative status in tissues of manganese superoxide dismutase transgenic miceFree Radic Biol Med 2003 28(3):397-402. [Google Scholar]
[34]. Ito Y, Kajkenova O, Feuers RJ, Impaired glutathione peroxidase activity accounts for the age-related accumulation of hydroperoxide in activated human neutrophilsJ. Gerontol 1998 53(3):169-75. [Google Scholar]
[35]. Baskaran N, Manoharan S, Karthikeyan S, Prabhakar MM, Chemopreventive potential of coumarin in 7, 12-dimethylbenz[a]anthracene induced hamster buccal pouch carcinogenesisAsian Pac J Cancer Prev 2012 13(10):5273-79. [Google Scholar]
[36]. Manoharan S, VasanthaSelvan M, Silvan S, Baskaran N, Singh AK, Vinoth Kumar V, Carnosic acid: A potent chemopreventive agent against oral carcinogenesisChem Biol Interact 2010 188(3):616-22. [Google Scholar]
[37]. Manoharan S, Kavitha K, Senthil N, Renju GL, Evaluation of anticarcinogenic effects of Clerodendron inerme on 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesisSingapore Med J 2006 47(12):1038-43. [Google Scholar]