Spinal anaesthesia is the anaesthesia of choice for surgery below the umbilicus due to relative ease of administration, fast onset, effective sensory and motor blockade with spared spontaneous respiration, low cost, safety especially in patients with full stomach, absolute relaxation of the intestines and abdominal wall facilitating surgery, eliminating the need for intubation, reduced blood loss, and earlier return of intestinal motility [1]. Spinal anaesthesia also has disadvantages, like haemodynamic disturbances, unsuitable for psychologically disturbed patients, inability to last for the duration of prolonged surgery, and failed spinal block. The complications associated with it, are total spinal or high spinal anaesthesia, Postdural Puncture Headache (PDPH), urinary retention, and waist and back pain [2].
Different adjuvants like opioids, adrenergics, GABA agonist, N-methyl-D-aspartate receptor (NMDA) antagonist, calcium channel antagonist, cholinesterase inhibitors have been used to prolong spinal anaesthesia, with the reduced postoperative analgesic requirements [3–5]. Also, these agents help to allay the fear and anxiety of the patient by their sedative effects.
The α-2 adrenergic agonists are being increasingly used as adjuvants as they provide sedation, analgesia, hypnosis and sympatholysis without causing respiratory depression. Although previous studies have shown significant prolongation of the duration of the sensory and motor blockade with intrathecal addition of α-2 adrenergic agonists on local anaesthetics and hence, synergistic interaction between the two [6–10]. However, there is limited literature regarding effects of intravenous α-2 agonists on the duration of spinal anaesthesia with ropivacaine.
Midazolam, with fast onset and short recovery time is a near ideal supplemental sedative that provides effective anxiolysis, predictable depth of amnesia, a rapid and clear headed recovery, with minimal side effects and no evidence of cumulation.
In this randomized single-blind placebo-controlled clinical study, we assessed the effects of intravenous dexmedetomidine infusion on subarachnoid block by ropivacaine in patients undergoing lower limb surgeries. The haemodynamic changes, duration of spinal anaesthesia as well as sedation and postoperative analgesia were observed and analyzed. To highlight dexmedetomidine’s analgesic effects, a comparison was made with midazolam and placebo (normal saline).
Materials and Methods
The study was conducted at Government Medical College, Patiala, Punjab, India, from year 2011-2014. The study protocol was approved by our Institutional Medical Ethics Committee, and written informed consent was obtained from each patient. Sixty patients, age ranging from 20-60 years, classified as American Society of Anaesthesiologists’ (ASA) physical status I–II and undergoing lower limb and lower abdominal surgeries under spinal anaesthesia were included. Exclusion criteria included refusal by the patient, use of any opioid or sedative medications in the week prior to surgery, a history of alcohol or drug abuse, known allergy to any of the test drugs or contraindication to spinal anaesthesia (e.g., coagulation defects, infection at puncture site, pre-existing neurological deficits in the lower extremities), and cardiovascular, respiratory, neurological, psychological, hepatic, or renal disease. After intravenous insertion of an 18-G catheter in the operating room, all patients were pre-loaded with 500 ml of lactated Ringer’s solution. Monitors included electrocardiography, non-invasive blood pressure measurement, and pulse oximetry to measure peripheral oxygen saturation (SpO2). The patient was placed in the lateral/sitting position and dural puncture was performed at the L3-L4 interspace using a standard midline approach with a 23-G Quincke needle. Ropivacaine 0.5% (3 ml) was injected intrathecally, the patients turned supine and received oxygen 4 l/min via a venturi mask throughout the procedure. This was immediately followed by a random allocation of the patients into one of the three groups. Randomization was carried out based on case registration (provided by hospital) numbers of patients with every third patient in each group.
Group D (n=20) received dexmedetomidine (a loading dose of 1 μg/kg over 10 minute followed by maintenance dose of 0.5 μg /kg/hr in form of infusion); Group M (n=20) received midazolam (loading dose of 0.05 mg/kg, followed by infusion at the rate of 0.02 mg/kg/hr), and Group C (n=20) received physiologic saline.
Sensory blockade was assessed using pinprick along the mid-clavicular line. Its onset, level and duration was recorded. Recovery time for sensory blockade was defined as two-dermatome regression of anaesthesia from the maximum level. Motor block was assessed immediately after sensory block assessment using a Modified Bromage Scale (MBS) [11] (1= Complete block i.e., unable to move feet or knees; 2= Almost complete block i.e., able to move feet only; 3= Partial block i.e., just able to move knees; 4= Detectable weakness of hip flexion while supine i.e., full flexion of knees; 5= No detectable weakness of hip flexion while supine; 6= Able to perform partial knee bend). Its onset and duration was recorded. Motor block duration was the time between MBS-2 to return of muscle power till MBS-5. Postoperative pain was assessed by the patient using the visual analogue scale (VAS; 0 = no pain; 10 = worst possible pain) at 4, 8, 12, and 24 hour. Patients with a VAS score of 4 or more received diclofenac 75 mg intramuscularly and the study was ceased thereafter in them. The time for the first request of postoperative analgesia and the number of patients who required supplemental analgesia were recorded. Level of sedation was assessed at 5, 10, 15 and 20 minutes according to the 5 point sedation scale [12] (0 - fully awake, 1 - Slightly drowsy, 2 - Asleep but easily arousable, 3 - Fully asleep but arousable, 4 - Sleeping and not arousable).
Baseline Pulse Rate (PR), blood pressure (systolic, diastolic and mean), oxygen saturation (SpO2), and Respiratory Rate (RR) were recorded. Intraoperatively, the vitals were recorded after every five minutes for 30 minutes after injection, thereafter every 10 minutes throughout the surgery. Hypotension (defined by a decrease in Mean Arterial Pressure (MAP) below 20% of baseline or systolic pressure<90 mmHg) was treated with intravenous mephentermine 5 mg. Bradycardia (HR<60 beats/minute) was treated with intravenous atropine 0.6 mg. The presence of any complication in the preoperative and postoperative periods was noted, particularly in relation to respiratory or cardiovascular problems, nausea or vomiting, and headache.
Statistical Analysis
The data was entered into Microsoft excel spreadsheet. The categorical data was expressed in terms of rates, ratios and percentage and continuous data was expressed as mean±Standard Deviation (SD). The data were analyzed statistically using SPSS version 17.0 computer software. One-way ANOVA (single factor) for data analysis and Tukey’s post-hoc test was applied. Categorical data were analyzed using the Chi-square test. The p-values <0.05 were considered to indicate statistical significance.
Results
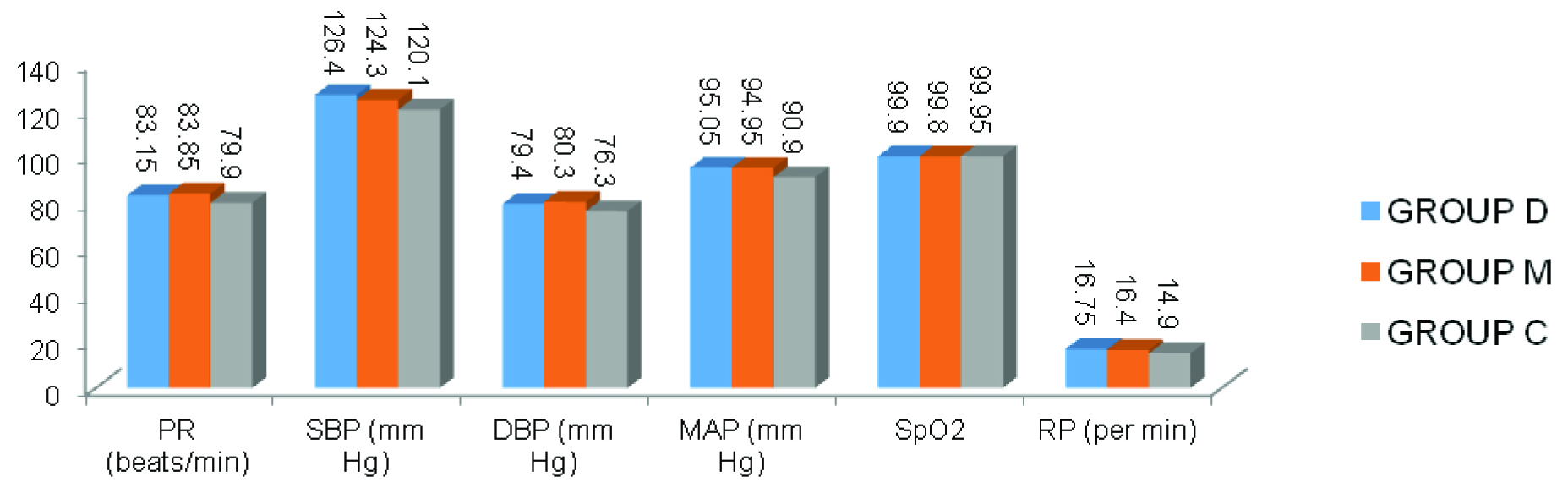
Spinal anaesthesia was successful in all patients, and all patients completed the study. Patient characteristics were similar among the three groups. No statistically significant difference was found between groups regarding demographic data and haemodynamic parameters [Table/Fig-1,2].
Demographic and intraoperative data.
| Variables | DexmedetomidineGroup D | MidazolamGroup M | SalineGroup C | p-value |
|---|
| Age (Years) | 33.40±9.98 | 36.35±12.97 | 32.75±10.39 | 0.559 |
| Weight (Kgs) | 68.80±8.33 | 65.60±9.57 | 64.60±5.49 | 0.229 |
| Height (Cms) | 172.03±7.74 | 169.05±5.74 | 167.98±3.16 | 0.086 |
| Gender (M:F) | 15:5 | 14:6 | 16:4 | 1.00 |
| 1.00(Dv/sM) |
| 0.716(Mv/sC) |
| 1.000(Dv/sC) |
| Duration of surgery (Minutes) | 82.5±13.72 | 72.5±19.70 | 74±21.13 | |
| Baseline parameters: |
| Pulse Rate (Beats/min) | 83.15±6.77 | 83.85±5.99 | 79.90±7.89 |
| Systolic Blood Pressure (SBP) (mm Hg) | 126.40±6.54 | 124.3±6.46 | 121.60±6.73 |
| Diastolic Blood Pressure (DBP) (mm Hg) | 79.40±6.68 | 80.30±8.11 | 76.30±7.63 |
| Mean Arterial Pressure (mm Hg) | 95.05±6.45 | 94.95±7.13 | 90.90±6.89 |
| SpO2 (%) | 99.90±.308 | 99.80±.410 | 99.95±.224 |
| Respiratory Rate (/minute) | 16.75±1.682 | 16.40±1.392 | 15.60±1.429 |
Preoperative haemodynamic parameters.
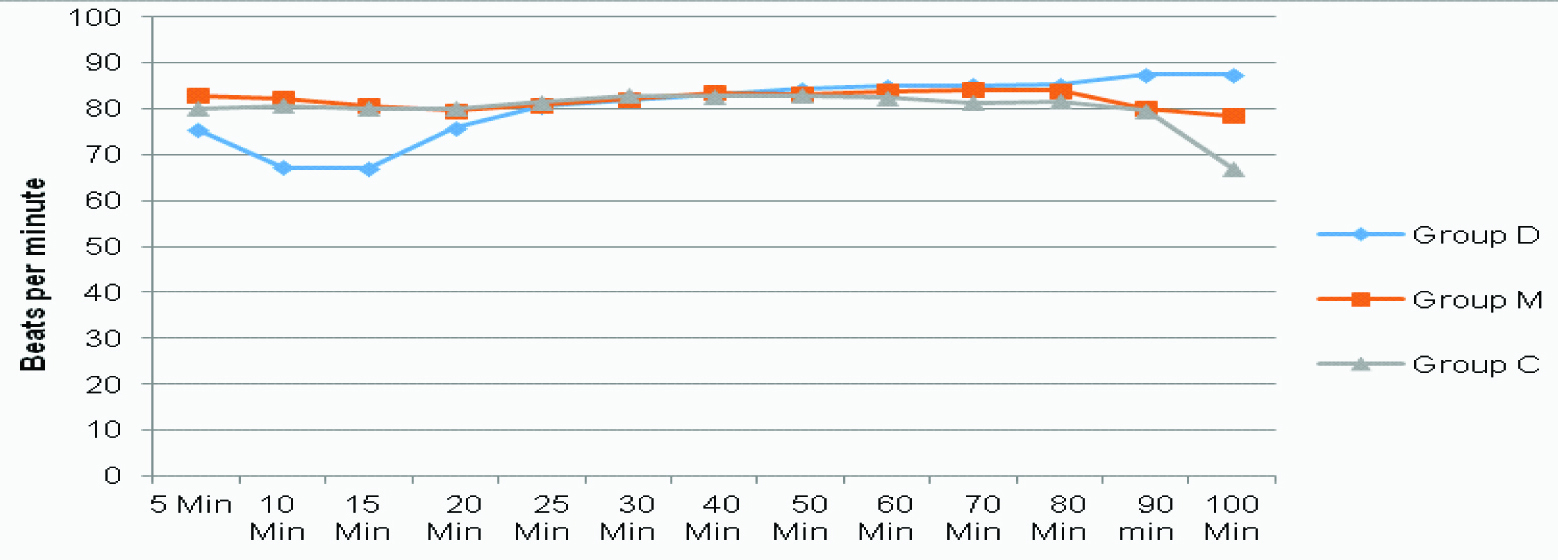
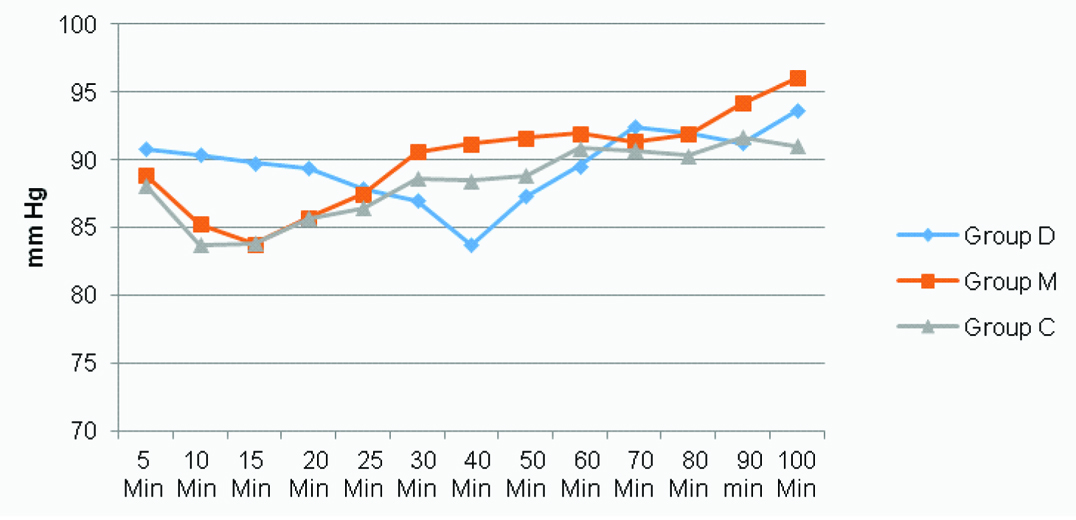
PR was found to reduce in Group D significantly greater than that in Group C for the first 15 minutes [Table/Fig-3,4] (p<0.05). Blood pressure was seen to fall, with mean arterial pressure significantly lower in Group C than in Group D at 15th minute. MAP-values were lower in Group D at the 40th minute, showing a gradual fall of blood pressure in this group [Table/Fig-4,5] (p<0.05). SpO2 and respiratory rate were found to be comparable all throughout the surgery.
Pulse rate for all three groups at different time intervals intraoperatively.
The p-value for haemodynamic parameters.
| Parameter | p-value | Significance |
|---|
| PR | 0.166 | NS |
| SBP | 0.078 | NS |
| DBP | 0.218 | NS |
| MAP | 0.100 | NS |
| SpO2 | 0.334 | NS |
| RR | 0.055 | NS |
ANOVA test applied.
Mean Arterial Pressure (MAP) for all three groups at different time intervals intraoperatively.
Comparison of the groups regarding the time to achieve peak motor and sensorial blockade revealed no statistically significant difference (p-value=0.08). The time (mean±SD) to reach peak sensory level was similar in the three groups [Group D, (6.13±0.96 min); Group M, (6.5±1.16 min); Group C, (5.88±1.12 min)]. The times to achieve peak motor blockade were (7.33±0.97 min) in Group D, (8.05±1.02 min) in Group M and (8.28±1.86 min) in Group C (p>0.05). There was no statistical significant difference amongst the three groups with respect to the upper level of sensory block achieved, with the median (range) peak sensory level, according to the pin-prick test, being T6 (T6-T10).
Median sedation levels were 3, 3 and 0 in Group D, M and C respectively. The time for sensory regression of two dermatomes in Group D (208±19.36 min) was significantly greater than that in Group M (177±15.25 min) (p<0.001) and Group C (177±17.8 min) (p<0.001). The duration of sensory block between the midazolam and saline groups was not statistically different [Table/Fig-6], (p-value 1.00). The time for complete regression of motor blockade in Group D (190.25±13.81min) was significantly much longer than that in Group M (136.50±17.54 min) (p<0.001) and C (149.60±18.82 min) (p<0.001). In Group D, five patients received mephentermine, while this happened to four patients in Group M and three patients in Group C respectively. This difference between groups was not statistically significant (p = 0.73). Whereas, eight patients in Group D received atropine, three patients in Group M and one patient in Group C was administered atropine, which was statistically significant (p=0.017).
Duration of block in each group.
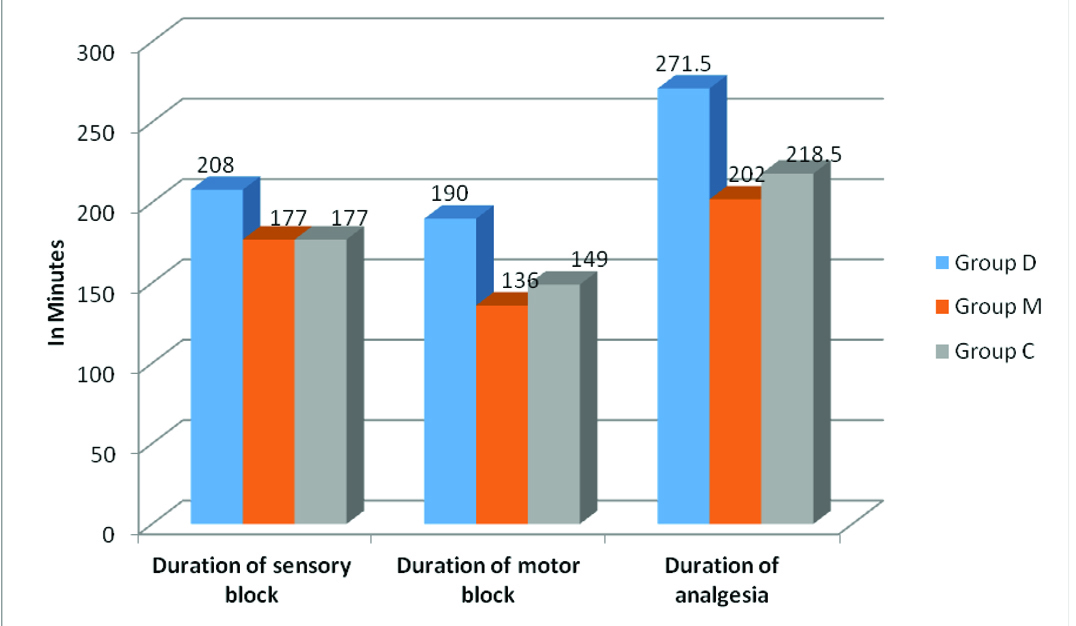
Time to first request for postoperative analgesia was longer in the dexmedetomidine group (271.50±21.83 min) than in the midazolam (202±25.05 min) and saline groups (218.50±38.01 min) (D v/s M and D v/s C: p<0.001) [Table/Fig-6,7].
The p-value for duration of analgesia.
| Group | p | Significance |
|---|
| D v/s M | 0.000 | HS |
| M v/s C | 0.236 | NS |
| D v/s C | 0.000 | HS |
Tukey’s Post Hoc analysis
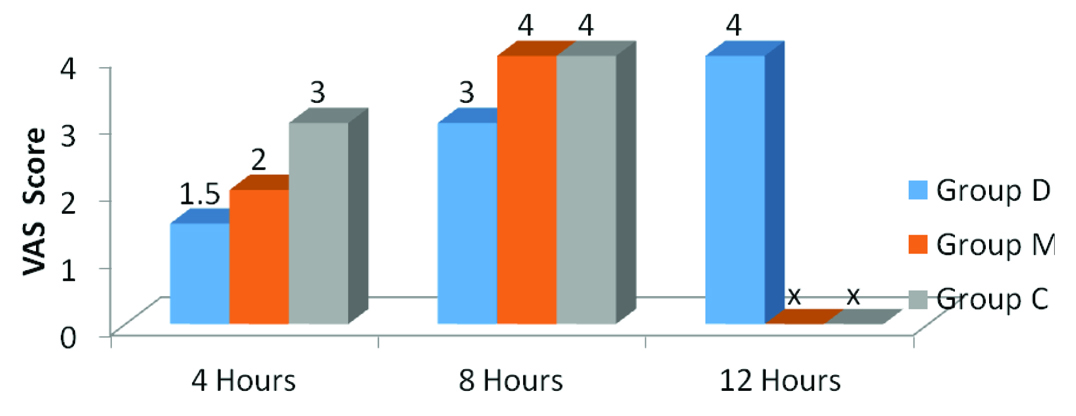
The VAS pain scores (recorded at postoperative 4, 8, 12, and 24 hours) revealed that Group M and C patients attained a VAS score of 4 earlier (in ≤8 hours) than patients in Group D (in ≤12 hours), hence, terminating the study in Group M and C earlier [Table/Fig-8]. Evaluation of the groups regarding complications revealed that urinary retention, vomiting, and respiratory depression were absent in all the groups. Whereas, shivering was seen to occur in 3, 2 and 1 patient in Group D, M and C respectively (p=0.57), Nausea was reported in 4, 3 and 3 patients in that order (p=0.887). Incidence of dry mouth was significantly higher in Group D (30% patients) versus Group M and C (5% patients in each group).
Post-operative VAS score.
(x = study terminated)
Discussion
There are many advantages of spinal anaesthesia, but the short duration of the local anaesthetic during prolonged surgery can be a problem. By prolonging the duration of spinal anaesthesia longer surgical interventions can be carried out. To prolong the duration of spinal anaesthesia, various additives such as opioids, neostigmine and also, vasoconstrictive agents such as epinephrine, phenylephrine and clonidine have been used as neuraxial analgesic adjuvants [13–15]. In very recent studies, intravenous dexmedetomidine administration is also shown to have synergistic action with local anaesthetics in prolonging the sensory and motor blocks, with good sedation effect and haemodynamic stability [6,16,17]. It helps to improve the quality and duration of spinal block and also provide postoperative analgesia. Also, sedation is frequently required during regional anesthesia. Propofol, midazolam, clonidine, and dexmedetomidine are frequently used for this purpose [18,19].
Our results indicate that intravenous dexmedetomidine significantly prolonged both the duration of sensorial blockade, and the time for complete reversal of motor blockade, though did not affect the onset time of sensorial block. This was in consensus to the study done by Elcıcek K et al., and Kaya FN et al., [20,21]. In addition, dexmedetomidine increased the time until first request of analgesic for postoperative pain relief and decreased the requirement of supplemental analgesic. It also provided sedation comparable to midazolam infusion. It has been reported that the α-2 agonists used in regional anaesthesia alter the characteristics of anaesthesia by inducing vasoconstriction [22,23] potentiating the blockade of C-fibres, or augmenting the effects of local anaesthetics by positively affecting slow retrograde axonal transport along the nerve at the spinal cord level [24,25].
The incidence of hypotension after spinal anaesthesia has been reported to be 30–40%, and this has been attributed to sympathetic blockade [13,26]. The incidence of reduced mean arterial pressure after dexmedetomidine infusion has been found to be 14, 17, 23, and 27 percent at infusion doses of 0.25, 0.5, 1.0, and 2.0 μg/kg, respectively [27]. In our study, the mean arterial pressures were found to be significantly lower in Group C than in Group D at 15th minute, whereas the fall in MAP in Group D occurred at the 40th minute, implying a gradual fall in this group. The incidence of mephentermine-requiring hypotension was found to be 25%, 20% and 15% in the dexmedetomidine, midazolam and control groups, respectively. This has been attributed to the spinal anaesthesia reaching maximum sensorial levels, and secondary to this hypotension is augmented by addition of the hypotensive effects of dexmedetomidine. The low incidence of hypotension has been attributed to provision of sufficient preoperative hydration to the patients.
In the literature, the incidence of bradycardia after spinal anaesthesia has been reported to be 10–15%. The incidence of reduced HR after dexmedetomidine infusion has been reported to be 25% [27]. In our study, we found significant reduction in HR in Group D for the first 15 minutes, compared with Group M and C. Besides, the incidence of bradycardia in Group D was found to be 40%, whereas, it was 15% and 5% in Groups M and C respectively. This has been attributed to the bradycardia- inducing effect of dexmedetomidine.
In studies performed with dexmedetomidine, the intended level of sedation has been reported to be achieved at doses of 0.2–0.7 μg/kg/h. Sedation has been also reported to be deepened with increasing doses [28,29]. In our study, deeper sedation was induced in Group D and M than in Group C, from which we have concluded that dexmedetomidine has the advantage of eliminating the need for extra sedative agents. One of the main objectives in using sedative agents is that respiratory depression should not occur. In previous studies it has been shown that the α-2 adrenergic agonists cause no or minimum respiratory depression [30]. In our study, respiratory depression was not observed in any of the patients.
The incidence of shivering after spinal anaesthesia has been reported previously to range between 10 and 40% [31,32]. In Group D we observed shivering in 15% of the patients, whereas, in Group M and C, the incidence was 10% and 5% respectively, which is in agreement with literature reports. In our study, the extent of nausea and vomiting were 15 and 0%, respectively, which is in agreement with literature reports. Complications such as voiding difficulty, vomiting and respiratory depression were not observed in any of our patients. Incidence of dry mouth was, however, significantly higher in Group D (30%) in comparison to Groups M and C (5% each).
Limitation
There are certain limitations of this study. The sample size was small. It could have been better with larger sample size. Secondly, older age group (>60 years) have been excluded from the study so effect of drug on older age group and their cardiovascular status was not studied.
Conclusion
It is seen that intravenously administered dexmedetomidine prolonged the duration of sensorial and motor blockade, provided sufficient sedation equivalent to midazolam, and had few side effects. We therefore, believe its application during spinal anaesthesia is appropriate, although heart rate needs to be monitored cautiously.
ANOVA test applied.
Tukey’s Post Hoc analysis