In adults, malignant astrocytic tumours are an aggressive form of Central Nervous System (CNS) tumours, glioblastoma multiforme being the most common among them [1]. Patients with these tumours have a poor prognosis and low survival rates [2]. Despite advances in surgical procedures and radiation and chemotherapeutic regimens, these tumours are still a cause for high mortality.
VEGF is a potent pro-angiogenic cytokine upregulated in malignant glial tumours [2,3]. In glioblastoma, angiogenesis is mostly driven by hypoxia dependent and independent mechanisms [3–7]. The proangiogenic role of VEGF in glial tumours has been well documented in other countries [1,3]. These studies demonstrated the upregulation of VEGF, thus, anti-VEGF modalities and therapies have been developed. In the Indian population, however, this association has not been evaluated. In this study, we analysed the expression of VEGF in malignant astrocytomas and oligodendroglioma and determined whether the expression pattern in our population is similar to that observed in other studies.
Materials and Methods
This is a prospective study approved by the Institute Ethics Committee for Human Studies at JIPMER. We obtained formalin fixed, paraffin embedded glial tumour biopsies {astrocytoma and oligodendroglioma (not otherwise specified)} from 60 patients (55% female; age range: 18–67 years (mean = 38.1 years) during a period of two years (2014 and 2015) from the Department of Neurosurgery, JIPMER, Puducherry, India. Informed consent was obtained from each patient.
All biopsies were characterised for tumour type and grade, according to the parameters established by the latest World Health Organization classification of brain tumours [8]. Of the 60 cases, 15 (25%) were classified as Grade II (diffuse astrocytoma- 12; oligodendroglioma – 3), 15 (25%) as Grade III (anaplastic astrocytoma – 2; anaplastic oligodendroglioma – 13) and 30 (50%) as Grade IV (glioblastoma).
Grade I tumours were excluded from the study as they are circumscribed benign tumours, in contrast to other gliomas which are mostly infiltrating and have tendency towards progression to malignancy. Also, the grade I tumours follow a distinctive molecular pathway unlike their malignant counter parts.
Immunohistochemistry: VEGF expression was analysed using immunohistochemistry. Sections were deparaffinised in xylene and then washed with alcohol; endogenous peroxidase was quenched using 3% H2O2 in methanol for 10 mins. The sections were then rehydrated in distilled water, and the slides were treated for nonenzymatic antigen retrieval in citrate buffer, pH 6.0 at 110 °C for one hour. They were then incubated with anti-VEGF primary antibody (rabbit polyclonal antibody prediluted in Phosphate Buffered Saline (PBS); BioGenex, San Ramen, CA, USA at room temperature (23 °C) for one hour in a humidified chamber. The slides were rinsed with Tris buffer and incubated with secondary antibody (Dako, Carpinteria, CA, USA) for 40 mins. They were rinsed again with Tris buffer and then incubated with Diaminobenzidine (DAB) for 15 mins. They were counter stained with haematoxylin, and then dried and mounted with di-n-butyl phthalate in xylene.
Scoring system: We used the scoring system for VEGF expression as proposed by Raica et al., as follows [9]:
SCORE 0, negative;
SCORE 1, weakly positive in less than 10% of tumour cells;
SCORE 2, weak to moderately positive in 10–50% of tumour cells;
SCORE 3, strong or moderately positive in more than 50% of tumour cells.
Statistical Analysis
The data were analysed using SPSS 19.0 software (IBM SPSS Inc., Chicago, IL, USA). Between-group VEGF expression levels and scores were compared using the Chi-square test. A p-value of <0.05 was considered statistically significant. The p-value was tested for two sided hypothesis.
Results
VEGF expression in astrocytoma and oligodendroglioma: VEGF expression was analysed staining with anti-VEGF antibody. While two (3.33%) cases showed no staining with the antibody, 58 cases (96%) showed positive staining for VEGF. Protein expression was detected in microvessels adjacent to endothelial cells along with the tumour cells in a few cases. VEGF expression was localised in the cytoplasm of tumour cells.
VEGF expression was analysed in various tumours. On the basis of the intensity of the staining [Table/Fig-1], two cases (3.33%) showed negative staining for VEGF, 10 cases (17.2%) showed weak staining, 17 cases (29.3%) showed weak to moderate staining, and 31 cases (53.457%) showed moderate to strong staining.
Expression of VEGF across the grades.
| Diagnosis | Negative | Weak in<10% oftumour cells | Weak to moderate in 10–50% of tumour cells | Moderate to strong in >50% of tumour cells | Total | p-value |
|---|
| Grade II | 0 | 7 | 4 | 4 | 15 | 0.012 |
| Grade III | 0 | 1 | 6 | 8 | 15 |
| Grade IV | 2 | 2 | 7 | 19 | 30 |
| Total | 2 | 10 | 17 | 31 | 60 |
Chi-square test was used to evaluate the association between VEGF Score & Grade of the tumour
Analysis of VEGF expression across tumour grades: Expression of VEGF was 100% in Grade II-diffuse astrocytoma (12/12), Grade II - oligodendroglioma (3/3) and Grade III tumours-anaplastic astrocytoma (2/2) Grade III – anaplastic oligodendroglioma (13/13), and 93.33% in Grade IV tumours-glioblastoma (28/30). The expressions of VEGF in tumours across various grades according to the VEGF scoring are shown in [Table/Fig-1]. We obtained a p-value of 0.012 (<0.05), which is significant.
Various expression details of VEGF in diffuse astrocytoma-Grade II, anaplastic astrocytoma-Grade III, glioblastoma multiforme-Grade IV have been displayed in [Table/Fig-2,3 and 4].
IHC- staining – VEGF cytoplasmic positivity – diffuse astrocytoma-grade II- 200x magnification – Score: 1.
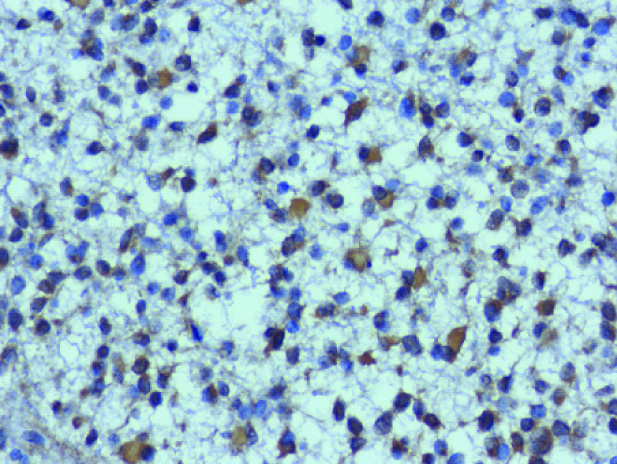
IHC- staining – VEGF cytoplasmic positivity – anaplastic astrocytoma-grade III-200x magnification – Score: 2.
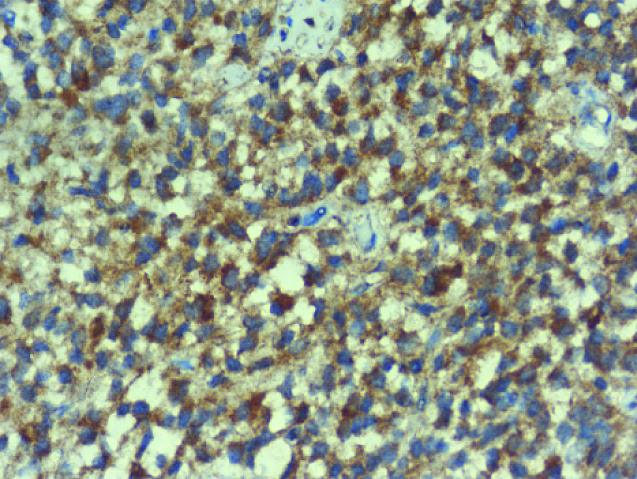
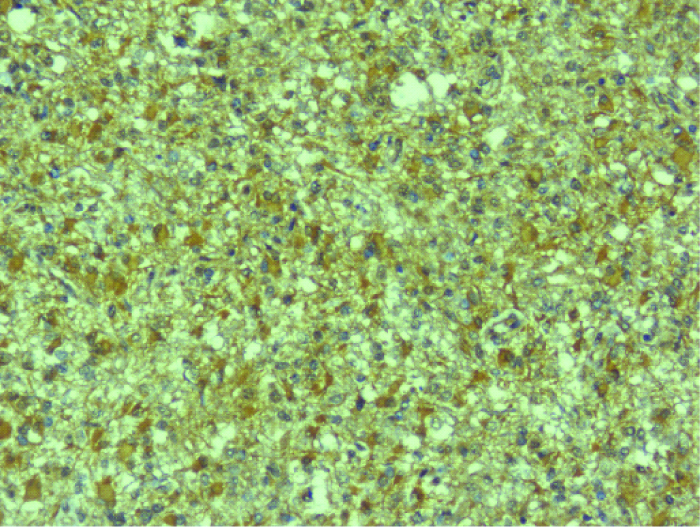
IHC- staining – VEGF cytoplasmic positivity –glioblastoma multiforme-grade IV-200x magnification – Score: 3.
Discussion
In the present study, we observed a higher incidence of Grade IV-glioblastoma cases than that of other grades as this is a tertiary care centre. VEGF expression positively correlated with the grades of tumour. A previous study elucidated that, VEGF expression, possibly upregulated by Phospho STAT3 (pSTAT3) in an invasion related signalling pathway regulated by the glioma cells, may be attributed to tumour invasion [10]. The same study found that expression of VEGF was positive and highly variable in the neoplastic cells which ranged between 12%-95%. However, the positivity was assessed in glioblastoma cases only [10]. In addition, over expression of VEGF was associated with the formation of peritumoural oedema along with cell invasion in these tumours [10]. In malignant gliomas, VEGF is responsible for inducing tight junction disruptions and neovascular endothelial cell dysfunction [11–13]. In vivo studies in mouse models and glioblastoma cell lines have also shown that, transcriptional up-regulation of VEGF and its secretion is mediated by Interleukin 6 (IL-6) and dependant on STAT3 [14].
Gupta et al., have also documented significant increase in VEGF expression in high grade gliomas in comparison with the lower grades and concluded stating that increased angiogenesis contributes to astrocytoma tumourigenesis in high grade tumours [15]. Erdamar et al., have also noticed similar patterns of VEGF expression which also correlated with histological grades in malignant astrocytomas [16]. Another study demonstrated consistent expression of VEGF in tumour cells of high grade gliomas and concluded that angiogenesis is closely associated with induction of VEGF in the tumour vasculature [4].
Although, there are many molecular markers for the diagnosis of glioma, the discovery of novel therapeutic markers is essential for patient management. In this regard, VEGF serves as a novel entity for designing anti-VEGF treatment regimens; however, these are in preclinical trials in USA and other countries [17–19]. Increased VEGF expression correlates with the degree of malignancy in gliomas [20,21].
Limitation
The expression of VEGF in these tumours plays a significant role. However, the association of different other factors like expression of VEGF-R, p-STAT3, micro-vascular density, peritumoural oedema etc with VEGF expression has not been addressed.
Conclusion
VEGF plays a role in neovascularisation in tumour cells. It may have an important role in the molecular mechanisms of tumourigenesis, thus, facilitating cell migration and proliferation, endothelial cell survival, and microvascular tube formation. VEGF has proven to be useful in cancer therapy owing to its pivotal role in angiogenesis. Therefore, studies at the genetic level by using sensitive and specific molecular techniques with larger cohorts are warranted. Among the Indian population, this was the first study in which the association between VEGF and tumour progression of astrocytomas and oligodendrogliomas was examined. The association of tumour progression with other molecular markers, such as VEGF-R and p53, and cell proliferative index need to be assessed to design effective therapeutic regimens that ultimately aim at personalising therapy.
Chi-square test was used to evaluate the association between VEGF Score & Grade of the tumour