There is a renewed interest in studying the neuroanatomical basis of psychiatric conditions, in the face of scant validated data available [1]. Neuroimaging provides for understanding of the biological abnormalities that under-pin psychiatric disorders apart from ruling out any organic disease of the brain.
Magnetic Resonance Imaging (MRI) is accepted as an important part of the comprehensive initial evaluation protocol in all psychiatric patients. Many studies utilizing MRI have been undertaken focusing on differences in grey matter volume, cortical thickness, curvature, surface area and white matter volume and ventricular volume measurements, in mental disorders. Only few studies have tried to look into pituitary changes in psychiatric conditions [1–7].
People who are in an acute phase of a psychiatric disorder show Hypothalamic–Pituitary–Adrenal (HPA) axis hyperactivity, but the precise underlying central mechanisms are unclear [2]. Pituitary gland being a master endocrine gland is the crux of the HPA axis. It is imperative to look for changes in the pituitary gland as part of the HPA axis activation in acute phases of psychiatric diseases.
To assess the pituitary gland volume variations in patients presenting with new onset acute psychiatric illness in comparison with age and gender matched controls by using MRI.
Materials and Methods
This was a prospective study undertaken in a tertiary care hospital, between October 2014 and September 2015. Fifty consecutive patients presenting with new onset acute psychiatric illness as diagnosed from the psychiatric department using ICD 10 diagnostic criteria, were recruited from the psychiatric outpatient clinics and were subjected to MRI of the pituitary gland. The sample size was estimated using the precision based sample size calculation formulae. The study was carried out with prior ethical approval and informed consent of all individuals enrolled. Patients with any known structural neurological disease, history of electroconvulsive therapy during the 6 months before the scan and other contraindication to MRI were excluded.
A total number of 50 healthy age and gender matched volunteers were recruited as controls. However, individuals with first degree (blood relation) relative known to be suffering from psychiatric illness or individuals already on antipsychotic medication were excluded.
Every case and control thus enrolled was subjected to MRI of Brain using 1.5 T scanner (Magnetom Avanto, Siemens Medical Systems). A High resolution Multiplanar T1 weighted sequence was utilised with parameters as mentioned in [Table/Fig-1].
High resolution Multiplanar T1 weighted MRI Protocol.
| Slice Thickness | 0.9 mm |
| Acquisition plane | Sagittal |
| Time of Repetition | 1180 milliseconds |
| Time to Echo | 4.4 milliseconds |
| Flip Angle | 15 degrees |
| Matrix Size | 256x256 |
| Field of view | 24x24 cm |
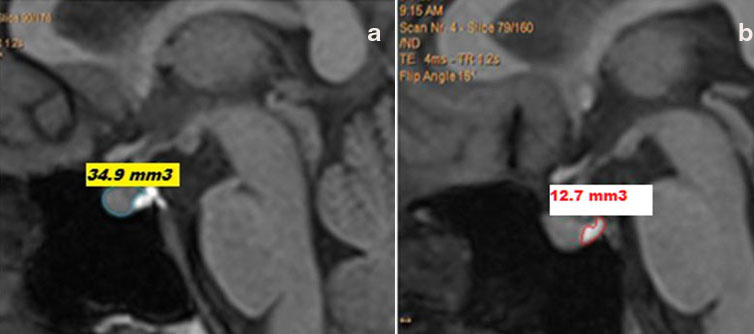
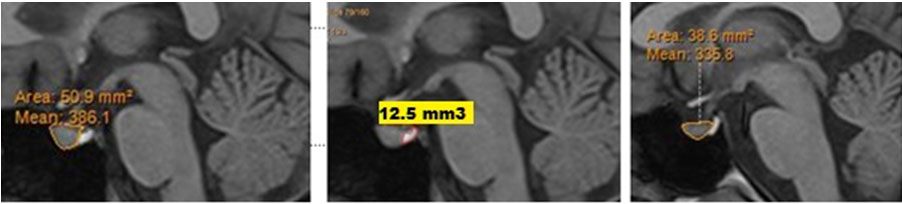
Pituitary gland was traced in all sagittal slices, where it was visualized. The pituitary stalk was excluded from the tracings. Anterior Pituitary and Posterior Pituitary bright spot were measured separately in each slice [Table/Fig-2]. Volume of the pituitary (in cm3) was calculated by summing up areas for all relevant slices and multiplying by a factor of 0.9, as slice thickness of images was 0.9 mm using the inbuilt software in the MRI system. No external or additional software was utilised for pituitary volume measurements.
T1 weighted sagittal images depicting (a) Anterior pituitary gland area and (b) Posterior pituitary gland area in the slice.
Means and standard deviations of the pituitary volume measurements were calculated out of the data. Initially, test of homogeneity of linear regressions between pituitary volumes of cases and controls, with age as the covariant variable, was done using Microsoft Excel regression analysis tool. Analysis of Covariance (ANOVA) was done to compare the significance of differences between Pituitary Volumes in Controls and Cases using Microsoft Excel Utility software for ANOVA (Version 2010).
Results
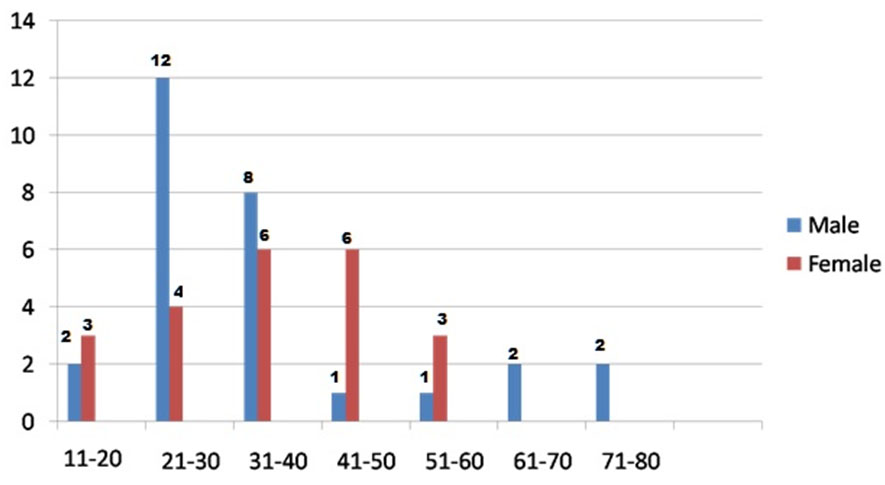
A total of 28 males and 22 females were enrolled as cases. The mean age of them was 36.3 years with a standard deviation of 14.9 years. The mean age of males was 35.1 years (standard deviation = 17.3 years) and females was 37.9 years (standard deviation = 11.4 years). The cases were classified based on their ages, into various class intervals, from 11-20 years to 71-80 years. Maximum number of cases were of the age groups 21 to 30 years (16) and 31 to 40 years (14) [Table/Fig-3].
Clustered bar diagram of age and gender distribution of cases.
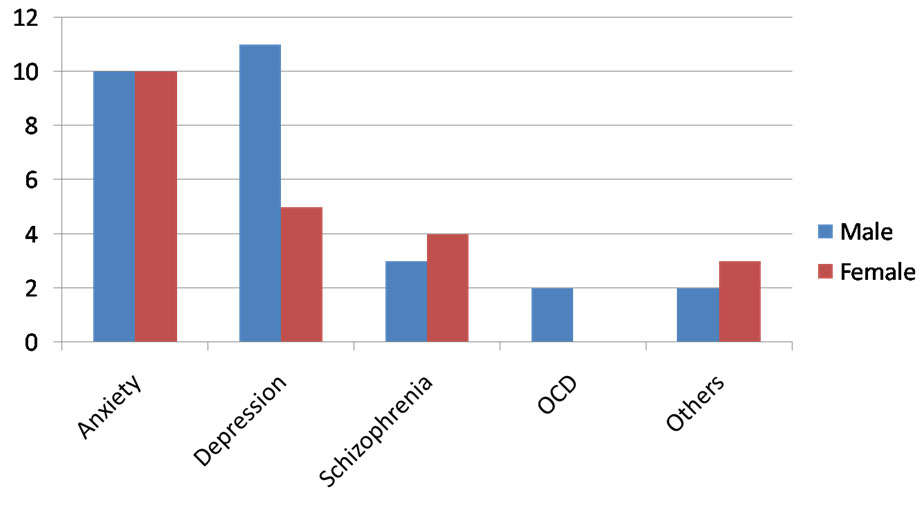
Cases were also divided into broad groups based on their Psychiatric diagnosis in consultation with the Department of Psychiatry. A significant number of cases fell into the category of Anxiety and Depressive psychosis [Table/Fig-4]. There were 20 cases diagnosed as anxiety related psychiatric illness. The second largest group included people diagnosed as Depressive psychosis.
Clustered bar diagram of psychiatric diagnosis profile – (Number of patients on y axis).
A test of homogeneity of linear regressions between pituitary volumes of cases and controls with age as the covariant variable showed significant correlation of slopes (f = 0.53, p=0.468).
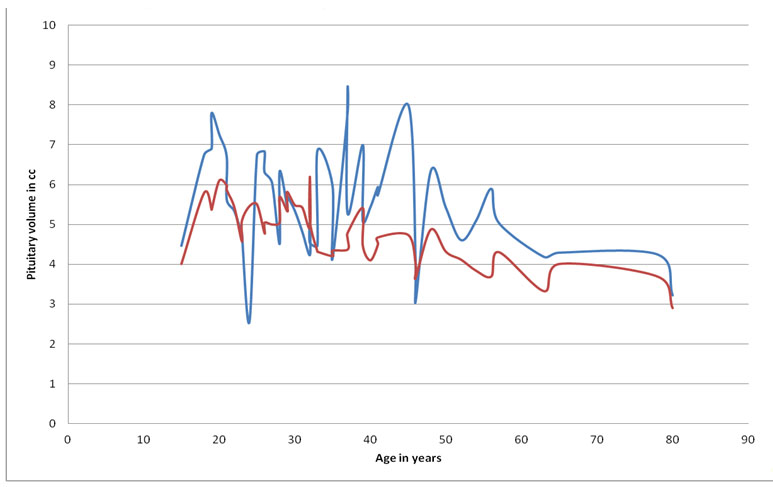
There was a significant difference in pituitary volume between the Controls and Cases (ANOVA, f=15.56, p=0.0002). The mean pituitary volume of Control group was 4.78 cm3 whereas it was 5.52 cm3 in cases. Pituitary volumes were 15.36% (0.73 cm3) higher than controls in cases [Table/Fig-5].
Stacked line diagram of comparison of pituitary volume between cases and controls. The blue line indicates pituitary volumes in cubic centimetres (cc) in cases and the red line indicates pituitary volumes in cubic centimetres in controls.
On further analysis it was found that the difference in the volumes of the Posterior Pituitary gland between Cases and Controls was insignificant (ANOVA, f=0.3, p=0.588). The cases had significantly higher anterior pituitary volume than controls (ANOVA, f=14.01, p=0.0003).
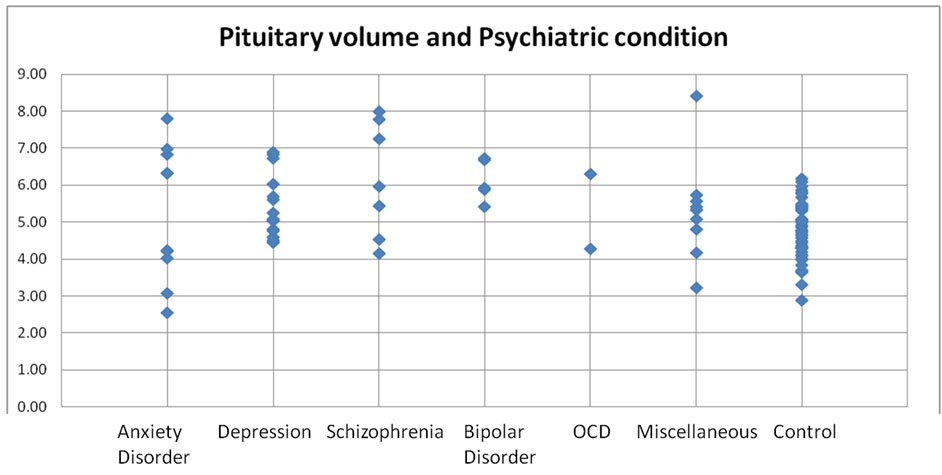
On analysis of pituitary volume in broad categories of psychiatric diagnostic profile [Table/Fig-6], it was noted that, there was a wide range of pituitary volumes exhibited by most of the groups, and the control group showed a much narrow range of dispersion of pituitary volumes.
Stock chart of comparison of pituitary volumes between various psychiatric conditions (acute phase) with controls.
However, none of the cases or controls enrolled in the study showed any abnormality in the MRI scans of the brains. MRI scans of all the cases and controls showed that the pituitary stalks were central in location on coronal reformatted images. None of the cases showed deviated pituitary stalks.
Discussion
The pituitary gland regulates HPA axis activity by secreting adrenocorticotrophic hormone, which in turn stimulates the adrenal gland to produce cortisol [2]. There is a possible role of this HPA axis in the pathogenesis of psychiatric conditions. In this study, patients with fresh onset acute psychiatric illness had larger pituitary volumes than healthy volunteers [Table/Fig-7]. This difference was significant irrespective of age. This finding is in concordance to many studies done previously.
A 19-year-old female with history of recent onset auditory hallucinations and delusions of grandeur and persecution: (a) Mid sagittal image of the T1 weighted MPR sequence at initial MRI with anterior pituitary size; (b) Mid sagittal image of T1 MPR sequence with posterior pituitary size; (c) Mid sagittal image of T1 weighted sequence in a 19-year-old healthy female control.
In a study done by Pariante CM et al., 17 out of the 24 patients with acute psychosis (71%) had pituitary volumes that were larger than the mean of the control group by an average of 10% and women had larger pituitary volumes than men in the group [3]. In the present study 37 out of 50 patients (74%) showed larger pituitary volumes by an average of 15.4% of mean of the control group. Also, in the whole sample, women had larger pituitaries than men.
A total of 11 of the 16 (69%) patients in the present study, with depression had higher pituitary volumes than controls. Krishnan KR et al., found that depressed patients had larger pituitary gland volumes than age and sex matched controls [4]. The study also found that there was significant difference in the anterior pituitary sizes only, which is in concordance with the present study. No significant correlation of posterior pituitary volumes was found in either of these studies.
Sassi RB et al., had found decreased pituitary volume in patients with bipolar disorder [5]. In contrast to this, the present study revealed higher volumes in 4 out of 5 (80%) patients with bipolar disorder.
MacMaster FP et al., studied pituitary gland volume on MR, in adolescent and young adult bipolar and unipolar depression and concluded that pituitary glands are enlarged in adolescents with mood disorders compared to controls [6]. They also found that healthy young females have larger pituitary glands than males, but such a difference was not evident in individuals with unipolar depression or bipolar disorder. In the present study the healthy female controls had higher pituitary volumes than their male counterparts.
Atmaca M et al., found smaller pituitary volume on MRI, in a group of patients with Obsessive Compulsive Disorder (OCD) [7]. They also showed that both men with OCD and control men had smaller pituitary volume compared to women with OCD and control women, respectively. Both the patients, in the present study, with OCD had larger pituitary glands than their controls.
It is suggested that the increased pituitary volume in patients with acute onset of psychiatric illness is due to activation of the Hypothalamic Pituitary Adrenal (HPA) axis. Sachar EJ et al., have examined the HPA axis in people with an acute episode of psychosis and have found hyperactivity of this hormonal system [8]. Krishnan KR et al., and Axelson DA et al., interestingly, described both HPA axis hyperactivity and increased pituitary volume in patients with severe major depression [4,9]. Pariante CM and Miller AH in 2001 and again Pariante CM in 2003 have interpreted in these patients as showing a lack of negative inhibitory feedback by circulating glucocorticoid hormones on the HPA axis, especially at the level of the pituitary (glucocorticoid resistance) [10,11].
Mineura K et al., found that increased size and number of corticotrophs and increased pituitary volume are present also in people with a lack of negative inhibitory feedback by circulating glucocorticoid hormones due to Addison’s disease [12].
Therefore, glucocorticoid resistance may be present in people with acute onset of psychiatric illness. As glucocorticoid resistance is a common correlate of stress-induced HPA axis activation in animals and humans, the findings of this study could be explained by an activation of the stress response [13]. Such activation could be due to the distress caused by the acute episodes of mental ailments, to an increased biological susceptibility to daily life stress or to an increased level of independent stressors leading to the psychotic episode or to all these causes [8,14,15].
The effects of age and gender on pituitary volume in our sample are consistent with previously published studies [5,16].
The pituitary volume was calculated by measuring serially the areas of the anterior and posterior pituitary gland separately in the sagittal images of a multiplanar T1 sequence. The anterior and posterior pituitary gland are better distinguished on sagittal images. This was in contrast to many other previous studies where it was a possible limitation to distinguish between anterior and posterior pituitary volumes on coronal images [3,5,9,17].
Future studies on similar, larger samples would clarify whether there are differences in pituitary volume across psychiatric diagnoses.
Limitation
Pituitary hormonal assays require sophisticated laboratory equipment and are expensive. Hormonal levels were not measured in this study due to financial constraints. So the proposed relationship between pituitary hyperplasia and hyperactivity of the HPA axis remains speculative. We also cannot exclude that pituitary hyperplasia in acute psychiatric illness is due to increased function of pituitary cells secreting hormones other than adrenocorticotrophic hormone, such as growth hormone and prolactin; levels of these hormones are also elevated by stress.
The pituitary gland was traced manually on every slice of MRI. This could be a possible limitation in spite of exercising due care for more accuracy. Software based volume measurements of the gland may yield better results. However, software based measurements have their own inherent drawbacks like inaccurate delineation of glands, leading to erroneous results.
In the present study, the number of cases under each individual psychiatric diagnosis was less, though the overall sample size was calculated based on precision based sample size calculation formulae.
In addition, there are no accepted reference values for pituitary volumes, especially in Asian population, for comparison. This could have been a drawback. In the present study, for each case, an age and gender matched control was selected, to overcome this limitation.
Conclusion
Pituitary volumes are significantly higher in patients presenting with acute onset of most psychiatric illness in comparison with healthy individuals of same age and gender. HPA axis overactivity has been postulated to be the cause of larger pituitary glands in such patients. Further studies are required to ascertain the biochemical and hormonal changes in these patients and also to predict the risk of developing psychiatric manifestations based on these stress response assessment.