Since the ancient past, man has used the plants to treat common diseases even before the discovery of the microbes. Herbs were utilized by the mankind as alternative medicines for treatment of various diseases due to their presumed trustworthiness, efficacy, safety and low price [1]. There is an increase in consumption of herbal medicines by the people of developing countries because of their belief that these products are natural; hence, they are relatively safe for the treatment of diseases. Apart from this there is also a false belief that natural products and herbal remedies cause less damage to the human body than the synthetic drugs [2]. The herbal preparations though believed to be safe, may contain impurities, combination of many toxic compounds, contaminants, microbes and rarely heavy metals [3,4]. After the administration of a particular chemical substance into a biological system there may be a chance of different type of interaction which can lead to adverse outcomes, sometimes even fatal. Because of these reasons toxicological studies have become mandatory to assess the safety of new drugs and herbal compounds which are under development. Published data about adverse reactions and toxicity are the key source for the regulatory safety assessment of natural herbal products [5]. As a part of new drug safety assessment, the pharmaceutical manufacturers are performing a series of toxicity tests which involves acute, sub-acute and chronic toxicity tests. Acute oral toxicity is routinely carried out as the first step to screen the safety and to evaluate the toxicity of compounds [6].
Materials and Methods
Collection and identification of plant material: Fresh leaves of C. roseus were collected during September to December, 2014 from the campus of Browns College of pharmacy, Khammam dist, Telangana state, India. Leaves were authenticated by the professor of Botany, SR and BGNR Govt Degree College, Khammam, Telangana. The experiment was done in the month of January 2015.
Preparation of ethanol extract: The plant leaves were dried in shade for six weeks and they were pulverized into coarse powder by using mortar and pestle. The powder was again ground into fine powder by using electric grinder and subjected to hot solvent extraction in Soxhlet apparatus using 98% ethanol (ethanol was collected from the central store of NRI Medical college, Guntur, Andhrapradesh, India). The yield was 27.6 gm and extract was suspended in water and administered orally to experimental rats.
Experimental animals: A total of 25 female, non-pregnant wistar albino rats, weighing 180-200 gm were used for the study. According to OECD 420 guidelines only female wistar rats should be used as the female rats are more sensitive than the males. Albino rats were obtained from the central animal house and maintained at standard conditions of 25±3°C and 55% relative humidity with a 12 hour light/dark cycle. Water and commercial rat feed was provided ad libitum.
The present study was approved by the Institutional Animal Ethics Committee with the IAEC number 44/IAEC/NRIMC/2014-2015. The study has been carried out according to the OECD guidelines for Testing of Chemicals, number 420 [10].
A total of 25 female non-pregnant wistar albino rats were randomized into five groups of five rats each (n=5). First group was normal control group and received distilled water, second, third, fourth, and fifth groups were treatment groups and received 5, 50, 300 and 2000 mg doses of C. roseus extract. A sighting study was conducted by giving 5 mg of CRE to the first rat of second group. Within 24 hours as there was no mortality, main study was conducted by giving the same 5 mg of dose of CRE to the rest of four rats in the same group. Similarly sighting study and the main study were conducted in all the groups with varying doses of 50, 300 and 2000 mg in groups 3, 4 and 5 respectively.
A single oral dose was administered and the animals were observed for 14 days for toxic signs. Rats were observed for mortality, general appearance, skin and fur, eyes and nose, respiration, locomotor activity, tremors, salivation, sleep and coma.
On 14th day, the blood samples were collected by retro orbital puncture and biochemical analysis was done for liver profile (SGPT, SGOT), renal profile (urea, creatinine) and cardiac profile (creatinine phosphokinase, LDH) [11].
Same day all the treatment and control group rats were sacrificed by giving an overdose of ether and complete necropsy was done. Liver, kidney and heart were dissected and preserved by using 10% formalin for routine histopathological examination.
Statistical Analysis
Biochemical findings were tabulated and statistically analyzed. The analysis was done by using SPSS software and represented as mean±standard error. These values were analyzed by ANOVA followed by post ANOVA t-test (scheffe’s test) at 5% level of significance (p≤0.05.)
Results
Physical Appearance and Mortality
After five hours of administration of 2000 mg dose tremors and restlessness observed in the fifth group of rats. No signs of mortality observed in the test doses studied.
Biochemical Parameters
SGPT, SGOT, urea, creatinine, creatinine phosphokinase and LDH were assessed and expressed as Mean±SE. 5 and 50 mg doses had not produced any effect in comparison to control group. All the parameters were elevated by dose dependent manner with 300 and 2000 mg of test drug. It is depicted in the [Table/Fig-1].
Effect of graded doses of Catharanthus roseus extract on biochemical parameters.
| Group No | Parameter | Cardiac Profile | Renal Profile | Liver Profile |
|---|
| CK | LDH | Urea | Creatinine | SGOT | SGPT |
|---|
| NS1 | Mean | 333.6 | 360.6 | 9.58 | 42.6 | 88.6 | 35.2 |
| SE | 3.473 | 3.696 | 0.159 | 1.470 | 2.993 | 1.463 |
| TD-52 | Mean | 337.4 | 363.2 | 11.36 | 52.8 | 94.8 | 54 |
| SE | 4.545 | 2.818 | 0.294 | 3.153 | 3.878 | 1.517 |
| TD-503 | Mean | 341.8 | 368.8 | 13.72 | 59.2 | 99.6 | 56.6 |
| SE | 3.992 | 2.354 | 0.116 | 1.497 | 3.14 | 1.435 |
| TD-3004 | Mean | 373.6a | 387a | 15.32a | 75.2a | 115a | 71a |
| SE | 1.691 | 1.817 | 0.139 | 1.068 | 1.673 | 0.632 |
| TD-20005 | Mean | 476.8a,b | 490.4a,b | 23.08a,b | 83.4a,b | 196.8a,b | 90.6a,b |
| SE | 2.956 | 1.470 | 0.886 | 1.600 | 1.625 | 1.249 |
†a = significantly different from control group; †b = significantly different from TD-300 mg
Histopathological Changes
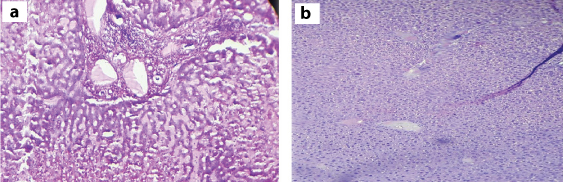
Liver: With 5 and 50 mg doses of test drug, no histopathological changes were observed in liver. With 300 mg of tested drug hepatocytes showed focal feathery degeneration. With the dose of 2000 mg there was moderate periportal inflammation and focal fatty change [Table/Fig-2a,b].
Histopathological changes of liver with 2000 mg of Catharanthus roseus extract: a) H&E stain liver -10x showing periportal inflammation with 2000 mg of plant extract; b) H&E stain liver -10X showing feathery degeneration, with 2000 mg of plant extract
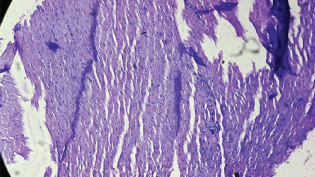
Heart: No histopathological change was observed even with 2000 mg. It is shown in the [Table/Fig-3].
Histopathological changes of heart with 2000 mg of Catharanthus roseus extract. H&E Stain. Myocardium – 10X showing normal muscle with 2000 mg of plant extract
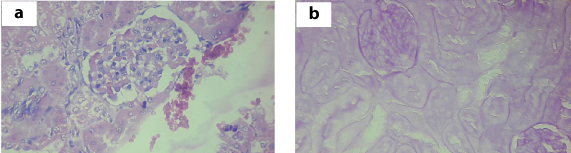
Kidney: There was no change observed with 5 and 50 mg doses. With the dose of 300 mg, there was focal thickening of the basement membrane and focal thickening of the tubular wall. With the dose of 2000 mg there was diffuse thickening of the glomerular basement membrane, increased mesangial matrix and focal thyroidization of the tubules. All the features indicate the chronic glomerulo nephritic changes in kidney. The histopathological changes in the kidney are shown in [Table/Fig-4].
Histopathological changes of kidney with 2000 mg of Catharanthus roseus extract: a) H&E stain kidney 40X showing increased eosinophliic proteinaceous material with 2000 mg of plant extract; b) PAS stain kidney 40X showing thickening of basement membrane with 2000 mg of plant extract
Biochemical changes are correlated with histopathological changes in liver and kidney. In spite of significant increase in cardiac enzymes, no histopathological change was observed in the heart.
Discussion
C. roseus plant is poisonous, if it is taken orally or if it is smoked. It is very poisonous in grazing animals [12]. Because of the above reasons and limited availability of the data on the toxic profile of the C. roseus; the present study was undertaken to evaluate the acute oral toxicity of ethanol leaves extract of C. roseus. Toxicity studies serve as an interface for hazard identification and safety evaluation [13]. Acute oral toxicity can be defined as adverse effect(s) which occur immediately or in a short period usually within 24 hours following the administration of test substance orally [14,15]. In the present study upto 2000 mg of plant extract, animals were safe and no death was observed within 14 days of administration. Some behavioural changes were observed with 2000 mg of oral administration of the test drug after five hours which include tremors, restlessness, loss of appetite etc. Similar changes were observed by Mohammed BM et al., [16]. According to them these behavioural changes can be attributed to the alkaloids of C. roseus and they may be responsible for the peripheral neuro toxicity. A similar study was conducted by the Kevin LY et al., which strongly supports the results of the present study in which acute exposure to methanol leaves extract of C. roseus in a dose range of 0.1 g to 1 g/kg to female rats did not produce any mortality and adverse effects even after 24 hour treatment [17].
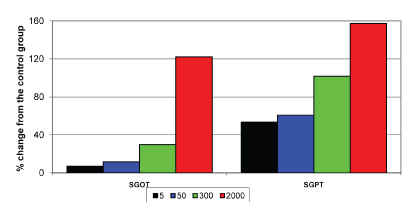
Biochemical changes were prominent at 300 mg and 2000 mg doses. With 300 mg dose the SGOT level was elevated by 29.8% and SGPT level by 101% from the basal values. A total of 2000 mg had increased the SGOT level by 122% and SGPT level by 157% from the basal values. These bio chemical changes are correlated with the histopathological changes and are shown in the [Table/Fig-5]. The results are not similar to the results of Sharma J et al., [18]. However, Sharma J et al., conducted the study in mice and the results had not shown any toxic effect or mortality even upto the dose of 10 gm/kg body weight within 24 hours of oral administration.
Effect of test drug on the biochemical parameters of liver in albino rats
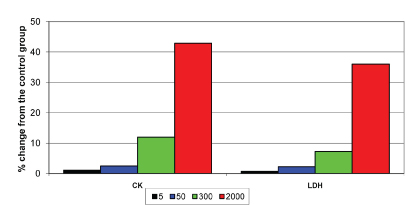
The cardiac profile had shown distinct changes at 300 mg and 2000 mg doses. Creatinine phosphokinase and LDH levels were elevated with both the test doses. The results are depicted in the [Table/Fig-6].
Effect of test drug on the biochemical parameters of heart in albino rats.
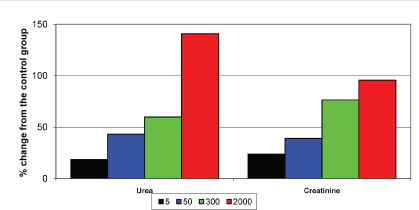
In the renal profile 300 mg had increased the urea level by 59% and creatinine level by 76% from the basal values. A dose of 2000 mg had increased the urea level by 140% and creatinine level by 95% from the basal values which is shown in [Table/Fig-7].
Effect of test drug on the biochemical parameters of kidney in albino rats.
Biochemical changes are correlated with the histopathological changes in liver and kidney. There was an increase in cardiac enzymes without any histopathological changes of heart. Based on these results liver and kidney damage can be anticipated at the doses of 300 mg. Upamanyu RA et al., described presence of several alkaloids, flavonoids, saponins etc., in the plant extract and may be responsible for damage of hepatocytes [19,20]. The present study results were strongly supported by the observations of Adekmoi DA et al., [21]. The study concluded that the extract have no toxic effects on the kidney and liver at the doses 200 mg/kg. A similar study was conducted by the Shetty Akhila J et al., the results of the present study are approximated with that study in case of renal profile changes [6]. According to them vinca alkaloids are the main cause of elevation of urea and creatinine and may be responsible for renal toxicity.
Limitation
The major limitation of the study is that, exact mechanism of action of C. roseus extract could not be explained. Specific markers were not used for evaluating the effect of plant extract on cardiac enzymes (except ethanolic extract).
Conclusion
Though C. roseus has been used for years as herbal medicine in traditional system. Caution should be exercised when using this product in high doses for prolonged periods. The ethanolic extract at doses higher than 300 mg can produce signs of biochemical and histopathological toxicity in liver, kidney and heart. It is recommended that lower doses than the studied ones should be used for treatment. It can be concluded from the study that herbal drugs may not be absolutely safe. So toxicity tests must be carried out before their use.