OSCC has been found to affect approximately 300,000 patients annually. In addition, it is a global health problem which constitutes upto 40% of all cancers [1,2]. Its aetiology is very complex and related to the type of carcinogen, its dose, frequency and application; susceptibility of the host and time period of interaction of carcinogen with host tissue. Chronic exposure to carcinogens such as tobacco, alcohol, oncogenic viruses and inflammation affect the individual genes and produce genetic alterations such as alterations of oncogenes and tumour suppressor genes, resulting in uncontrolled tumour growth [3].
Tobacco is the primary aetiological factor in OSCC development as its consumption exposes the oral epithelium to carcinogenic benzopyrene and nitrosamines which induce oncogenic transformation. It has been shown that these carcinogens up-regulate the expression of cytochrome P450 (CYP1A1 and CYP1B1) supergene family, a phase I detoxification system under in vitro and in vivo conditions [4,5]. CYP1B1 has been found to be over expressed in a wide array of human tumours as compared to their respective normal tissues. Transcriptionally activated CYP1B1 gene plays a crucial role in the bioactivation of chemically diverse tobacco related procarcinogens to reactive metabolites as a product of phase I reaction. These reactive cytochrome P450 metabolites are detoxified by phase II detoxification system enzymes [6]. Glutathione-S-transferease (GST) belongs to multigene family of phase II enzymes and provide protection against chemical stress and carcinogens by conjugating them with GSH and thereby eliminate it via GSH conjugate recognizing transport [7]. Accumulated data reveals that the association exists between impaired detoxification and carcinogenesis [8].
Moreover, tobacco consumption and smoke also exposes the oral epithelium to large amount of toxic Reactive Oxygen Species (ROS) such as Hydrogen peroxide (H2O2) and hydroxyl radicals, that can evade or overwhelm the antioxidant protective mechanisms of cells and tissues, and produce major interrelated impaired cell metabolism including DNA strand breakage, rises in intracellular free Ca2+, damage to membrane ion transporters and other specific proteins leading to disease process [9–11].
Prime target to free radicals attack are the polyunsaturated fatty acids in the membrane lipids, causing lipid peroxidation, has been found to be a major event in the production of pathophysiological alterations in various diseases including cancer [12,13]. Lipid peroxidation brings about the production of a number of reactive aldehydes including MDA. These reactive aldehydes bind to membrane proteins and alter their function, tonicity, permeability, rigidity and integrity, which in turn enhances carcinogenesis [12,14,15]. Antioxidant defense system, by virtue of antioxidant enzymes and antioxidants, plays a crucial role in scavenging free radicals. Total Antioxidant Activity (TAA) including co-operative action of other widely recognized non enzymatic antioxidants such as vitamin C, E, A, uric acid and albumin, have a crucial role in protecting the body from deleterious action of ROS and received much attention in preventing carcinogenesis. Depection in antioxidant reserve and overproduction of ROS lead to pathophysiological alteration responsible for development of various diseases including cancer [16–18].
Materials and Methods
The present study was hospital based case control study. In a period of 12 months (October 2012 to September 2013), a total 78 suspected OSCC patients visited to outpatient clinic in the Department of Oral medicine at Rajah Muthiah Dental College and Hospital, Chidambaram, Tamil Nadu, India, with oral disease complaint. A total of 37 patients did not meet the inclusion criteria and rest 41 subjects had histopathologically confirmed evidence of OSCC. A total of 20 OSCC patients were recruited in the study as patient group after complete screening and confirmation of OSCC by oral medicine physician, whereas, 21 subjects refused to participate. The presence of OSCC along with its grading was confirmed by histopathological investigation of the patient carried by oral pathologist. A general information or pre-experimental questionnaire regarding demographic information, family history and limited physical examination was completed from all the subjects.
Inclusion criteria: A total number of 20 OSCC patients (13 males and seven females) confirmed by histopathology and belonged to 30-50 years of age were included. The sample sizes were calculated by statistician of the college keeping principle, administrative issues, duration of the study, prevalence rate of the disease in study area and cost in mind [19].
To diminish any confounders developed by other types of oral diseases, only 20 patients with histopathologically confirmed OSCC (irrespective of their grading) were recruited. It could also be due to less number of OSCC patient visited in hospital outpatient department (OPD) in the study period. Among the 20 OSCC patients, 12 patients (three males and nine females) had family history of smoking and chewing tobacco. In addition, 13 patients were under medical treatment including supplementation of antioxidants and chemotherapy. They were also not excluded from the study because no supplement or antioxidant vitamin would be taken by them in the seven days before entry into the study. However, there was no restriction or withdrawal on the conventional anti-cancer drugs treatment.
Exclusion criteria : Patients, aged above 50 and below 30 years, with established cardiovascular complications, diabetes, pregnancy, lactation, obesity (BMI>25), hypertension (BP>120/80 mmHg), renal failure, liver disease, hypothyroidism, adenocarcinoma, sarcoma, precancerous lesions and other cancer conditions or who did not follow study instructions were also excluded from the study. In addition, patients under hormone replacement therapy and those with a history of infections and other oral diseases were excluded from the study.
A total of 20 age and sex matched healthy individuals (13 males, 7 females) from college staff and their family members were recruited as controls after taking their informed consent and proper oral check up by oral medicine physician. They had neither tobacco chewing or smoking habit nor any oral disease. The subjects having fasting glucose levels, serum transaminases, Blood Urea Nitrogen (BUN), creatinine levels beyond normal range were excluded from the control group.
The blood samples (6 ml) of both the study group subjects were collected in plain vial (2 ml for serum separation), EDTA vial (3 ml) and fluoride vial (1 ml) after overnight 12 hours fast. As per the selection criteria in each group, subjects were recruited in study only after obtaining their informed consent. Information regarding their demographic status, clinical history, family history and medications were noted down in detail. The ethical committee of Rajah Muthiah Medical College and Hospital, Annamalai University, approved the study protocol.
Haematological parameters such as haemoglobin, RBC and WBC count from the whole blood of the patients were analyzed by using the cell counter (cellinium 19 haematology analyzer) and general biochemical parameters such as fasting blood glucose, serum urea, serum creatinine and plasma lipid profile was estimated in semiauto analyzer using enzymatic kit method (Accurex Biomedical Pvt. Ltd.
Fasting blood glucose was estimated by glucose oxidase method. Glucose oxidase converts glucose to gluconic acid. In addition, Peroxidase (POD) produces hydrogen peroxide which oxidatively couples with 4-aminoantipyrine and phenol to produce red quinoneimine dye [20].
Serum urea was estimated by urease method which involves the splitting of urea into ammonia and carbon dioxide by the action of enzyme urease. Ammonia released in this reaction reacts with hypochlorite and phenolic chromogen to produce green colour. The intensity of colour was directly proportional to concentration of urea and measured at 578 nm [21].
Serum creatinine was estimated by kit method which involves the reaction of creatinine with alkaline picrate to produce orange colour. The intensity of colour was directly proportional to concentration of creatinine and measured at 492 nm [22].
Plasma cholesterol was estimated by enzymatic kit method which involves the conversion of cholesterol ester into free cholesterol and fatty acid by cholesterol esterase. In the second reaction, cholesterol oxidase acts on cholesterol and produce cholest-4-ene3-one and hydrogen peroxide. H2O2 oxidatively couples with 4-aminoantipyrine and phenol to produce red quinoneimine dye. This dye had absorbance maximum at 510 nm [23].
Enzymatic kit method was also used in the estimation of plasma triglyceride. Triglyceride was hydrolyzed by lipoprotein lipase to release glycerol which was converted into glycerol 3 phosphate by glycerol kinase. In addition, glycerol phosphate oxidase converts glycerol 3 phosphate into dihydroxy acetone phosphate and hydrogen peroxide. In presence of peroxidase, hydrogen peroxide oxidizes phenol chromogen to red colour compound. The intensity of colour was directly proportional to concentration of triglyceride and measured at 510 nm [24].
Plasma High Density Lipoprotein (HDL) was estimated by using phosphotungstic acid/Mg2+ which precipitates chylomicrons, Very Low Density Lipoprotein (VLDL) and Low Density Lipoprotein (LDL) fraction whereas HDL fraction remains unaffected in supernatant. Cholesterol content of HDL fraction was assayed using autozyme cholesterol [25].
Plasma LDL-cholesterol and VLDL-cholesterol levels were calculated by Friedwald’s formula [26].
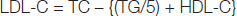
VLDL cholesterol = Total cholestrol – (HDL + LDL)
Serum Glutathione-S-transferase (GST) was estimated by 1-Cholro, 2,4-Dinitro Benzene (CDNB) method [27]. GST was estimated in incubation mixture of phosphate buffer and CDNB reagent. Reaction was started by adding GSH and serum followed by its measurement at 340 nm.
Erythrocyte MDA levels were measured as thiobarbituric acid reactive substances, after preparation of haemolysate. In this method, the heat induced reaction of MDA with Thio Barbituric Acid (TBA) in the acid solution forms a trimethine coloured substance, which was measured spectrophotometrically at 532 nm [28].
Plasma total antioxidant activity was estimated by the method of Karocevic D et al., which involve the formation of as the hydrogen peroxide react with standardized solution of iron EDTA complex. Hydroxyl radical causes release of TBARS by degrading benzoate which was suppressed by the antioxidants of blood plasma and spectrophotometrically measured at 532 nm [29].
CYP1B1 genotypic analysis by Polymerase Chain Reaction (PCR): Genomic DNA extraction was carried out from peripheral blood leukocytes using the modified salting out method of Miller et al., [30]. Analysis of CYP1B1 genotype was carried out by PCR-based restriction digestion method using specific primers:
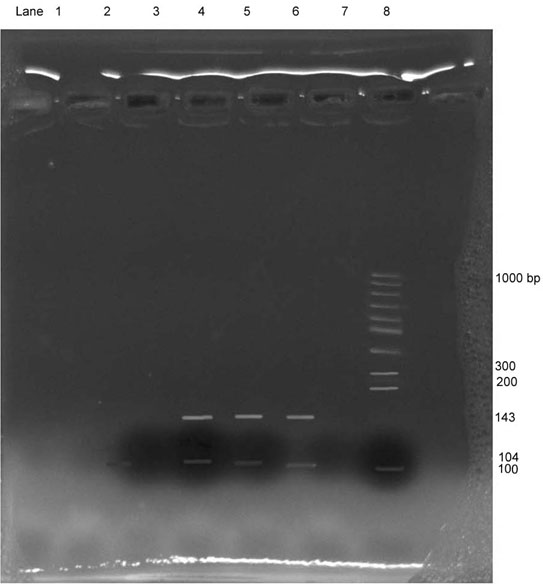
FP: 5’ACC AGC CCA ACC TGC CCT ATG T 3’, RP: 5’ GCT TCT TAT TGG CAA GTT TCC TTG GCT 3’. 50 μl of PCR reaction mixture contained 5 μl genomic DNA, 25 μl 2 X Master mix (Taq DNA polymerase, 1X Taq Buffer, MgCl2 and dNTPs), 5 μl of each specific primers (forward and reverse) and 10 μl ultra pure distilled water. PCR was carried out in thermal cycler of Applied Biosciences 2710 with an initial denaturation step at 95°C for five minute followed by 30 cycles, each consisting of 94°C for one minute, 60°C for one minute and 72°C for one minute, followed by a cycle of extension at 72°C for 10 minute. PCR product of 143 bp obtained after amplification was subjected to digestion with Eco571 enzyme which recognizes the sequence 5’CTGAAG (N16/N14) 3’. The digested fragments of approximately 104 and 38 bp were observed on 12% polyacrylamide gel electrophoresis. The results were interpreted based on the size and number of bands obtained in gel documentation system [Table/Fig-1].
CYP1B1 genotypic analysis by PCR in oral cancer patients and controls.
ECO571
Sample 1 : Negative control Sample 2 : Positive control
Sample 3 : Negative control Sample 4 : Positive case
Sample 5 : Positive case Sample 6 : Positive case
Sample 7 : Negative case Sample 8 : Molecular weight marker
Statistical Analysis
The data collected from patients and control were entered separately in Microsoft Excel sheet of windows 2007 and values were expressed as Mean±SD. The significance of mean difference between patient and control groups was compared by using Student’s t-test. The distribution of ‘t’-probability was calculated depending on ‘n’ and significance of test was obtained. The p-value <0.05 and <0.001 were considered as significant and highly significant, respectively. The p-value>0.05 was considered as insignificant. In addition, correlation analysis between aforesaid parameters was performed by using Pearson correlation test.
Results
Demographic profile and haematological parameters in the study group population were depicted in [Table/Fig-2]. In the present study, haemoglobin levels (p=0.0001; 22.98% low) and RBC count (p=0.0196; 31.57% low) were significantly decreased in oral cancer patients as compared to healthy controls whereas WBC count altered insignificantly (p=0.553) in patients group with respect to controls.
Demographic profile and haematological parameters in the study group population. (Mean±SD).
| S.No. | Parameters | Control group(n=20) | Patient group(n=20) | p-value |
|---|
| 1 | Age (years) | 41±5.1 | 43±5.3 | 0.2315 |
| 2 | Height (meter) | 1.63±0.29 | 1.61±0.26 | 0.819 |
| 3 | Weight (Kg) | 57.0±1.7 | 58.0±2.0 | 0.104 |
| 4 | BMI (Kg/m2) | 22.5±1.2 | 23.0±1.3 | 0.214 |
| 5 | Systolic blood pressure (mmHg) | 106.3±3.10 | 108.0±3.20 | 0.961 |
| 6 | Diastolic blood pressure (mmHg) | 76.0±2.25 | 76.2±2.38 | 0.7863 |
| 7 | Haemoglobin (gm%) | 12.14±1.18 | 9.35±0.72 | 0.0001 |
| 8 | RBC(millions/cumm) | 3.8±1.7 | 2.6±1.4 | 0.0196 |
| 9 | WBC count(cells/cumm) | 5.72±1.23 | 5.52±0.85 | 0.553 |
Where, p<0.1: Non significant; p<0.05: Significant
Plasma lipid profile and other biochemical parameters in oral cancer patients and healthy controls were represented in [Table/Fig-3]. Lipid profile contents and fasting blood glucose levels revealed insignificant variation (p>0.05) in oral cancer patients as compare to healthy controls which indicate that these parameters are not generally affected by the incidence of carcinogenesis. Similarly, renal function was also not affected by oral cancer as authenticated by insignificant alterations (p>0.05) in the levels of serum urea and creatinine in patients group as compared to controls.
Plasma lipid profile and other biochemical parameters in the study group population. (Mean±SD).
| S. No. | Parameters | Control group(n=20) | Patient group(n=20) | p-value |
|---|
| 1 | Fasting blood glucose (mg%) | 85.80±8.17 | 86.95±8.10 | 0.657 |
| 2 | Serum urea (mg%) | 28.95±4.20 | 32.15±4.52 | 0.258 |
| 3 | Serum creatinine (mg%) | 0.80±0.28 | 0.85±0.22 | 0.534 |
| 4 | Total cholesterol (mg%) | 187.55±24.17 | 179.60±13.04 | 0.203 |
| 5 | Triglyceride (mg%) | 97.15±7.76 | 93.15±9.46 | 0.152 |
| 6 | HDL-cholesterol (mg%) | 40.60±3.33 | 38.40±4.12 | 0.071 |
| 7 | LDL-cholesterol (mg%) | 115.60±14.50 | 108.30±16.41 | 0.144 |
Where, p<0.1: Non significant; p< 0.05: Significant
Marked alteration was observed in the levels of marker of oxidative stress and phase II detoxification enzyme as presented in [Table/Fig-4]. Serum GST activity was significantly reduced (p<0.012; 20.0% low) in patient group with respect to controls. Similarly, plasma Total Antioxidant Activity (TAA) levels were decreased significantly (p=0.0402; 24.28% low) in oral cancer patients as compared to healthy controls. On the other hand, erythrocyte MDA levels were increased significantly (p=0.0001; 37.95% high) in patients group as compared to controls. In addition, [Table/Fig-1] showed the expression of CYP1B1 genotype in study group subjects. It was observed that CYP1B1 gene was expressed in 17 patients (85 %) of oral cancer patients, which indicate that the expression of CYP1B1 is an important determinant of carcinogenesis.
Markers of oxidative stress and Phase II detoxification enzyme activity in oral cancer patients and healthy controls (Mean±SD).
| S. No. | Parameters | Control group(n=20) | Patient group(n=20) | p-value |
|---|
| 1 | GST (IU/L) | 4.10±1.18 | 3.28±0.67 | 0.012 |
| 2 | MDA (μmol MDA/ml) | 2.45±0.13 | 3.38±0.14*** | 0.0001 |
| 3 | TAA (m mol/L) | 1.40±0.15 | 1.06±0.70** | 0.0402 |
Where, p<0.1: Non significant; p<0.05: Significant; GST: Glutathione S transferase; MDA: Malondialdehyde; TAA: Total antioxidant activity
On performing correlation analysis, it was observed that alteration in serum GST activity was positively associated (p<0.05; r= 0.564) with reduction in plasma TAA levels and negatively related (p<0.001; r = -0.728) with erythrocyte MDA levels, an efficient marker of lipid peroxidation.
Discussion
Development of cancer in humans is a multistep process which involves a complex series of cellular and molecular changes mediated by a diversity of endogenous and exogenous stimuli [31]. In this context, use of tobacco is a well-established aetiological factor in the development of oral cancer and several metabolic enzymes have been investigated for their possible role in cancer susceptibility including members of CYP450 super family (CYP1A1 and CYP1B1). These enzymes are primary agents involved in oxidizing the carcinogens found in tobacco like polycyclic aromatic hydrocarbon, nitrosamines and arylamines [32]. It has been suggested that CYP1B1 plays more important role than CYP1A1 in metabolic activation of environmental procarcinogens [33]. Shimada T et al., also reported that human CYP1B1 plays an important role in tobacco related head and neck carcinogenesis as it catalyzes the oxidation of polycylic aromatic hydrocarbon into reactive metabolite of Phase I detoxification system [34].
In our study, it was observed that 85% of histopathologically diagnosed cases had expression of CYP1B1 which reflects that the CYP1B1 is an important determinant on carcinogenesis. Similar findings have been reported be Murray GI et al., in their study on regulation, function and tissue specific expression of cytochrome P450 CYP1B1 [35]. In addition, over expressed CYP1B1 protein has been detected in a variety of human tumours including those of the lung, brain, testis, breast, kidney and ovary [35,36]. Interestingly, Pradhan S et al., reported downregulation of CYP1B1 in oral tumour tissues as compared to their matched normal tissues and suggested that a level of caution should be observed for treatments based on CYP1B1 overexpression in tumours [37].
Phase II conjugation reaction generally follows phase I activation resulting in the transformation of xenobiotics in to water soluble compound that can be excreted through urine. Phase II reaction involves various enzymes such as GST which favours the elimination of carcinogen by conjugating it with GSH [38]. Song LL et al., also reported that serum GST is a major detoxifier of dihydrodiol and epoxide form of polycyclic aromatic hydrocarbon [7]. Depletion in GST activity leads to the retention of reactive carcinogen and induce carcinogenesis. In the present study, serum GST activity was also found to be significantly low (p<0.05) in OSCC patients which reflect its insufficiency in the prevention of cancer development. Consistent findings have been documented by Ma HL et al., in their study on OSCC patients. According to them, decreased GST expression in patients with OSCC and a lower GST expression indicate a poorer pathological differentiation grade in OSCC tissue samples [39]. However, conversely, Hirata S et al., showed progressive increase of GST activity in advanced stage of carcinoma [40].
Moreover, tobacco consumption not only exposes the oral epithelium to toxic carcinogens but also to toxic oxygen and nitrogen free radicals that can affect host antioxidant defense mechanisms followed by epithelial cells of oral mucosal membrane. Previous studies have also reported the elevated levels of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in oral precancer and cancer patients [9,11,31]. These free radicals are scavenged by antioxidant defense system of the body. In general, alteration in total antioxidant activity indicates the disturbance in the antioxidant defense system of the body due to oxidant overload.
In the present study, plasma TAA levels were decreased significantly in OSCC patients as compared to controls and positively correlated with GST, which elucidate the combined effect of reduced antioxidant defense system and insufficiency of phase II detoxification system enzyme in OSCC pathology due to augmented oxidative stress. Similarly, marked reduction in TAA levels in OSCC as well as in subjects with other inflammatory diseases have been well documented in earlier studies [13,41]. Depletion of antioxidant defense system leads to enhanced lipid peroxidation which is well characterized by attack of reactive oxygen species on phospholipid rich membrane of intracellular organelles (lysosomes) and epithelial cells of oral mucosa and thereby carcinogenesis [42]. In the present study, erythrocyte MDA levels were found to be significantly high (p<0.001) in OSCC patients with respect to healthy controls which authenticate the fact that lipid peroxidation plays a key important role in the carcinogenesis. Similarly, Manoharan S et al., also observed increased levels of MDA along with depleted antioxidant reserve in plasma and erythrocytes of oral cancer patients [43] Sabitha KE et al., also showed that enhanced production of MDA reacts with nucleic acid and contribute significantly in mutagenesis and carcinogenesis [44].
Limitation
The inability to follow up the subjects with OSCC, no criteria for selecting the cases according to the stages of cancer and inadequacy in sample size, due to paucity of time and limited resources.
Conclusion
On the basis of findings of present study and consistent findings of previous studies, it can be concluded that CYP1B1 expression and other oxidative stress variables are significantly associated with OSCC. Thus, measurement of GST activity, screening of CYP1B1 expression (if possible) along with total antioxidant status and cessation of tobacco use could not only limit the occurrence of OSCC but also help to decrease the incidence of OSCC as these markers are one of the conventional risk factor of OSCC. However, to validate the findings of the current study, multicenter study with large sample size and in different ethnic populations should be carried out to support the findings of the current observation from this study.
Where, p<0.1: Non significant; p<0.05: Significant
Where, p<0.1: Non significant; p< 0.05: Significant
Where, p<0.1: Non significant; p<0.05: Significant; GST: Glutathione S transferase; MDA: Malondialdehyde; TAA: Total antioxidant activity