The present study was conducted to determine the prevalence of epidemiology, antimicrobial susceptibility pattern and clinical outcome of S. maltophilia in the paediatric hospital setting in a tertiary care hospital in Delhi.
Materials and Methods
This was a retrospective study done over a period of eight months, October 2015 to May 2016. Data was collected from a hospital information management system. All non fermenting Gram-negative bacilli from any clinical specimen like pus, tracheal aspirate, blood, urine etc., were selected. The clinical samples were processed using standard microbiological procedures. Out of all the non-fermenters, S. maltophilia isolates were further selected. Final identification and antibiotic susceptibility of these isolates for levofloxacin and trimethoprim-sulphamethoxazole was performed by VITEK 2C system (Biomerieux, France).
The VITEK 2C is an automated microbiology system utilizing growth-based technology. It has colorimetric reagent cards that are incubated and interpreted automatically. A total of 16234 clinical specimens were received from our hospital in the microbiology laboratory between October 2015 to May 2016, with 2734 pathogenic bacteria isolated.
All the patients were followed up and their clinical outcome and average length of stay in the hospital was calculated. Average length of stay and mortality caused by S. maltophilia infection was compared with controls. Controls were taken as double the number of cases, were matched for age and sex with cases and were inpatients with infection other than S. maltophilia.
As the sample size was very small, statistics could not be employed. Ethical clearance was not required as it was a retrospective study. All the samples were routine samples and no special intervention was done.
Results
A total of 1339 gram negative bacteria were isolated, out of which 414 were non fermenters. Among the non fermenters, 23 (5.5%) were S. maltophilia. Out of the 23 isolates, 15 (65.2%) were isolated from blood, 4 (17.3%) were isolated from urine and tracheal aspirate each [Table/Fig-1].
Sample wise distribution of the S. maltophilia isolates.
| Sample | No.(%) |
|---|
| Blood | 15 (65.2) |
| Tracheal aspirate | 4 (17.3) |
| Urine | 4 (17.3) |
| Total | 23 |
Out of the 23 patients, 18 (78.2%) were males and 5 (21.7%) were females. Maximum patients were above five years of age (34.7%) followed by one month-one year (26%), one to five year (21.7%) and below one month of age 4 (17.3%) [Table/Fig-2]. Out of the 23 patients, 9 (39.1%) were admitted to wards, 5 (21.7%) were in ICU, 5 (21.7%) were seen in OPD and 4(17.3%) were in emergency [Table/Fig-3].
Demographic profile of the patients with S. maltophilia infection.
| Gender | No. (%) |
|---|
| Female | 5 (21.7) |
| Male | 18 (78.2) |
| Age | |
| <1 month | 4 (17.3) |
| 1 month-1 year | 6 (26.0) |
| 1-5 year | 5 (21.7) |
| >5 year | 8 (34.7) |
| Total | 23 |
Location wise distribution of the S. maltophilia isolates.
| Location | No.(%) |
|---|
| In-patients/wards | 9 (39.1) |
| ICU | 5 (21.7) |
| OPD | 5 (21.7) |
| Emergency | 4 (17.3) |
| Total | 23 |
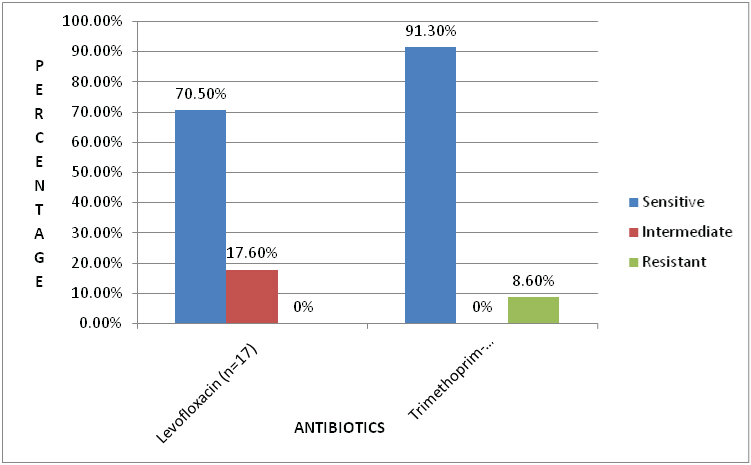
A total of 21 (91.3%) of strains were susceptible and 2 (8.6%) were resistant to trimethoprim-sulphamethoxazole. A total of 12 (80%) of strains were sensitive and 3 (20%) had intermediate susceptibility for levofloxacin. None of the strains were resistant to levofloxacin [Table/Fig-4].
Antimicrobial susceptibility pattern of the S. maltophilia isolates.
Maximum resistance was seen in the blood isolates followed by urine and tracheal aspirate [Table/Fig-5].
Sample wise antimicrobial sensitivity pattern of the S. maltophilia isolates.
| Drugs | Blood (n=15) | Trachealaspirate (n=4) | Urine (n=4) |
|---|
| Levofloxacin(Numerator/Denominator) | 80% | 75% | 100% |
| Trimethoprim-sulphamethoxazole(Numerator/Denominator) | 100% | 75% | 75% |
Numerator- Number of resistant isolates, Denominator- Length of stay
MIC value of trimethoprim-sulphamethoxazole ranged between ≤20 to ≥320 μg/ml and levofloxacin ranged between 0.12 to 4 μg/ml.
Average length of stay of patients with S. maltophilia infection was found out to be 23.3 days as compared to 44.8 days in controls. The average mortality of patients with S. maltophilia infection was found to be same as that of controls (35.2%) [Table/Fig-6].
Comparison of outcome of patients with S. maltophilia infection with those of controls.
| Variables | Cases (n=17) | Controls (n=34) |
|---|
| LOS (days) | 23.3 | 44.8 |
| Mortality | 35.2% | 35.2% |
LOS- Length of stay
Discussion
S. maltophilia is a Gram-negative bacterium generally considered as having low virulence, is becoming an emerging multi-drug resistant opportunistic pathogen in hospital as well as community settings, especially among immunocompromised hosts. Various risk factors associated with S. maltophilia infection include underlying malignancy, cystic fibrosis, cortico-steroid or immunosuppressant therapy, the presence of an indwelling central venous catheter and exposure to broad spectrum antibiotics [10,11]. There is paucity of information on the world wide prevalence of S. maltophilia infections in the paediatric population [11].
In our hospital S. maltophilia was found to be the third most common non-fermenters after Acinetobacter spp and Pseudomonas aeruginosa accounting for 5.5% of total non-fermenters. This finding was similar to that reported by Rit K et al., from Eastern India [12] and Jia W et al., from China [6].
Abdel-Aziz N et al., reports the total isolation of S. maltophilia among Gram-negative bacteria to be 1.5% which was comparable to our study (1.7%) [5]. Jia W et al., reports the total prevalence of S. maltophilia among the non-fermenters to be 10.1% [6] which was higher than that of our study. In another study from Eastern India, S. maltophilia represented 2.8% of total non fermenters [12].
In this study, S. maltophilia was most commonly isolated from blood (65.2%) followed by urine and tracheal aspirate each (17.3%). Jia W et al., reports maximum isolation from respiratory specimens [6]. Abdel-Aziz N et al., reports maximum isolation from urine sample followed by swabs and blood [5].
In our study, 78.2% isolates were from males as compared to 21.7% in females. Similar rates were seen in a study conducted in Karnataka, India [13]. Risk factors associated with S. maltophilia infections include immunosuppressive therapy, admission to the Intensive Care Unit (ICU), advanced age, prolonged hospital stay, long term antimicrobial therapy and surgical procedures [14]. In our study, 21.7% patients were admitted to ICU, 39.1% were in wards and rest were OPD patients. Among this population, we found maximum isolation in children more than five years of age followed by infants with one month to one year of age. To the best of our knowledge, this is the first study conducted in India in paediatric population.
Trimethoprim-sulphametoxazole is considered as the treatment of choice for S. maltophilia infections [11]. In our study, 70.5% strains were susceptible to levofloxacin, none were resistant and 91.3% strains were sensitive to trimethoprim-sulphamethoxazole and 8.6% were resistant. Our results were similar to a study done in South Africa in 2015 which reported 98.3% resistance to trimethoprim-sulphamethoxazole and 1.3% resistance to levofloxacin [15]. Abdel-Aziz N et al., reports 100% sensitivity for trimethoprim-sulphamethoxazole and 16.67% susceptibility to fluoroquinolones [5]. Another study conducted in Hungary reports 99% susceptibility to trimethoprim-sulphamethoxazole and 75% susceptibility to levofloxacin [16] Chawla K et al., reported 78.8% susceptibility for levofloxacin and 72.7% for trimethoprim-sulphamethoxazole [13]. Many studies have demonstrated the emergence of strains resistant to trimethoprim-sulphamethoxazole. Our study reports a lower resistance for both the drugs. A probable explanation could be that this study has been conducted on a relatively antibiotic naïve population.
Most infections caused by S. maltophilia were associated with severe morbidity and long-term, extensive ICU treatment. According to previous reports, the mortality rates vary between 14-62% [17].
Our study reports a mortality rate of 35.2% which is in accordance with the known data. A study conducted in India reports the mortality rate to be 21.2%, which was a little lower than our study [13]. The study conducted in Hungary reports the mortality rate to be 45%, however, the attributable mortality rate was 11%. In our study we did not calculate the attributable mortality rate.
In our study the average length of stay in patients with S. maltophilia infection was found to be 23.3 days as compared to 44.3 days in the control cases. However, the mortality rate was found to be same in both the groups (35.2%). As compared to the controls, the relative mortality of children infected with S.maltophilia was higher. Thus, concluding that S.maltophilia causes more serious infections in paediatric population leading to a higher fatality.
To the best of our knowledge, no study has been reported from India highlighting the prevalence, antibiotic susceptibility and clinical outcome of S. maltophilia in paediatric population. Rit K et al., and Chawla K et al., have done a similar study in adult population [12,13]. A few case reports have been reported from India. We provide a summary of all the cases reported from India for past decade [Table/Fig-7] [1,2,10,18–20].
Summary of case reports of S. maltophilia reported from India in the last decade [1,2,10,18–20].
| S. No. | Year | Age/sex | Diagnosis | Site of isolation | Antibiotics given | Outcome |
|---|
| 1 | 2009 | 30 year/male | Endophthalmitis | Vitreous fluid | Ciprofloxacin | Recovered |
| 2 | 2010 | Newborn/female | Early onset neonatal sepsis | Blood | Cefotaxime, Amikacin | Expired |
| 3 | 2010 | Newborn/female | Early onset neonatal sepsis | Blood | Piperacillin-tazobactam, Amikacin | Expired |
| 4 | 2012 | 44 year/male | Conjuctival ulcer | Conjuctival scraping | Moxifloxacin | Recovered |
| 5 | 2015 | 2 year/female | Sepsis | Blood | Amoxyclav, Antimalarials, Ceftriaxone | Recovered |
| 6 | 2015 | 9 year/female | Sepsis | Blood | Amoxyclav, Antimalarials, | Recovered |
| 7 | 2015 | 7 year/female | Sepsis | Blood | NA * | Recovered |
| 8 | 2015 | 60 year/male | End stage renal diseases | Blood | Levofloxacin | Recovered |
| 9 | 2015 | Newborn/female | Early onset neonatal sepsis | Blood | Levofloxacin, Ceftazidime | Recovered |
*NA-Not available
Limitation
There were many limitations in our study. First of all, it was a very short study, conducted over eight months. A larger study with better planning should be conducted to find out the current epidemiology and resistance burden. Secondly, the identification and antimicrobial susceptibility testing was done only by VITEK. The results should have been confirmed with manual MIC calculations as well. Thirdly, although patients were followed-up to find out the outcome of disease, but the clinical risk factors could not be evaluated. We plan to conduct a bigger study to overcome the limiting factors and to find out the actual burden of the resistant isolates and their clinical implications as well.
Conclusion
Many multi-institutional studies done on a worldwide level have confirmed that S. maltophilia is an emerging multi-drug resistant opportunistic pathogen in hospital and community settings, especially among immunocompromised hosts. Trimethoprim-sulphamethoxazole still remains the drug of choice in the general population. There is an alarming trend in resistance to previously known susceptible drugs such as ceftazidime, ticarcillin-clavulanate, and fluoroquinolones. A better understanding of the epidemiology, antimicrobial susceptibility profile and clinical outcomes of S. maltophilia is required, so as to control the increasing trend in isolation rate of this pathogen from various clinical samples. Regular surveillance and continuous monitoring should be done for better management of patients.
Numerator- Number of resistant isolates, Denominator- Length of stay
LOS- Length of stay
*NA-Not available