Introduction
Due to bioaccumulation and long half-lives, OCPs are ubiquitously detected in humans. In India several recent studies have reported the presence of OCPs such as HCH, endosulfan, DDT, aldrin and their metabolites in the blood samples of general population [1,2]. It is believed that OCPs bio-accumulates through food chain, stored in fat depot and release slowly into the circulation. Due to the occurrence of OCPs in considerably low level in human, they do not exhibit acute toxicity and are frequently ignored. Some of the OCPs due to their endocrine-disrupting capabilities, have been shown to be associated with infertility, preterm birth and cancer of reproductive organs [3,4]. Recently some of the OCPs have been shown to be associated with hypertension, CVD and stroke [5,6]. Despite their presence in considerably low level in humans, their biological effects may be hazardous for human health since these toxic molecules or their metabolites may interact with proteins, enzymes, receptors, transcription factors and signalling molecules, thereby altering their function and regulatory role. However, the mechanism leading to OCP-mediated ill effects is yet to be deciphered.
Several investigators have suggested that oxidative stress may be the potential mechanism behind tissue injury produced by exposures to OCPs as evidenced by enhanced lipid peroxidation, decrease in glutathione and antioxidant enzymes [5–9]. Pesticides can produce oxidative stress through different pathways like generation of ROS as a by-product of xenobiotic metabolism, impairment of mitochondrial membrane-bound electron transport, inactivation of antioxidant enzymes and depletion of free radical scavengers [6].
NADPH oxidase (NOX) is the main enzyme responsible for producing ROS as its primary function [7]. At physiological concentration ROS act as a signalling molecule, however at higher concentration it causes cellular dysfunction and induce apoptosis. Environmental exposure to lipophilic persistent organic pollutants such as biphenyl, dioxine and OCPs that accumulate in adipose tissue, has recently been found to be associated with the risk factor for CVD [10]. How these pollutants induce CVD remains to be elucidated. Oxidative stress has been implicated in CVD for a long time. Under conditions of oxidative stress, ROS attack biomolecules causing lipid peroxidation, protein cross-linking, mitochondrial and nuclear DNA damage resulting in mutations, protein denaturation and loss of enzyme and membrane pump function [11]. Also the cause-effect relationship of oxidative stress with cardiovascular diseases is yet to be elucidated.
NOX is the main enzyme responsible for superoxide generation in the endothelium and has been reported to exhibit profound effect on the bioavailability of NO. Therefore, it is possible that OCPs may alter the expression profile of NOX in the endothelium. Some of the OCPs have been shown to upregulate the expression of NOX in peripheral blood mononuclear cells (PBMC) and neutrophils [12].
Upregulated NOX generates increased amount of ROS in endothelial cell that may be associated with reduced NO bioavailability via uncoupling of eNOS [13]. Therefore, it may be possible that pesticides induce oxidative stress by increasing ROS production via NOX pathway and subsequently reducing the bioavailability of NO leading to endothelial dysfunction.
In view of this hypothesis the present study has been designed to find out the effect of four organochlorine pesticides in HUVEC in vitro regarding to ROS generation, NOX and eNOS expression.
Materials and Methods
This in vitro study was carried out at the Department of Biochemistry, University College of Medical Sciences during December 2015 to August 2016. In vitro study was carried out to find the effect of OCPs (β-endosulfan, aldrin, α-HCH and p,p’-DDE) on ROS generation, NOX and eNOS expression in primary endothelial cells (HUVEC).
Culture and Treatment of HUVEC: Primary endothelial cells were obtained (HiFiTM Umbilical Vein Endothelial cells, HUVEC, CL002-0.5, Himedia) and cultured in endothelial cell expansion medium (HiEndoXLTMAL517, Himedia, India) containing Media-199 supplemented with endothelial growth factors, 15% FBS and 1% antimycotic-antibiotics solution in T-25 culture flask in a humidified atmosphere of 5% CO2 at 37°C. Media was changed every 2nd day. Cells were sub-cultured with trypsin-EDTA solution on reaching the confluency of 70-80%. Action of trypsin was neutralized by addition of complete medium containing FBS. Cells reaching the 3rd passage with 70-80% confluency were used for experiments. The cell numbe drs were counted and viability of the cells was checked by cell counter (Invitrogen). The cultured cells were treated with four OCPs namely aldrin, β-endosulfan, α-HCH and p,p’-DDE at a concentration of 0.1 μM which is based on the average residue found in human blood. Ameliorating effect of anti-oxidant N-acetylcysteine (10 μM) on pesticides mediated effects on HUVEC was also studied. These compounds were obtained from Sigma-Aldrich, St. Louis, MO, USA.
Determination of Intracellular ROS: Intracellular ROS generation was assessed by using 2’, 7’-dichlorodihydrofluoresce in diacetate (H2DCFDA). Cells were treated for two hours at 37°C in the presence of 100 μM H2DCFDA (the stock solution was made in methanol). After the incubation time, cells were washed twice with cold phosphate buffered saline (1X PBS). An aliquot of 100 μl was incubated in a black 96-well plate, and relative fluorescence intensity was determined by spectrofluorimetry (λex = 488 nm, λem = 520 nm).
Determination of Endothelial Nitric-Oxide Production: Nitric oxide produced by HUVEC is measured indirectly via measurement of its breakdown product nitrite in the cell lysate by using Bioxytech nitric-oxide assay kit. Briefly, assay mixture containing 50 μl of culture medium, 35 μl of assay buffer, 10 μl of nitrate reductase and 10 μl of NADH was incubated at 37°C for 20 minutes. At the end of the incubation period the assay mixture was treated with 50 μl of Griess reagent (sulphanilamide and naphthalene-ethylenediaminedi hydro chloride). The absorbance at 540 nm was measured using a micro-titer plate.
NOX and eNOS expression in HUVEC Cells: Total RNA was extracted from HUVEC by Trizol (Invitrogen, Carisbad, CA). The extracted RNA had an OD 280/260 ratio between 1.9 and 2.0. cDNA was synthesised taking 1 μg of total RNA with Maxima first stand synthesis kit (Fermentas, Germany). Quantitative real time PCR (Rotor Gene Q, Qiagen) was performed to measure expression of mRNA for NOX and eNOS using SYTO9 fluorescent dye. The reaction mixture contained 4 μl template cDNA, 10 μl of PyroStart fast PCR master mix (Fermentas, Germany) and 10 pmol of each primer of both target genes and housekeeping gene. The sequence of each primer is as follows: NOX p47phox subunit (target gene) forward: 5’GCTCCCCACGGACAACCAGAC3’, reverse: 5’TCTTCTCCACGACCTCCACCAC3’ eNOS (target gene) forward: 5’GTGGCTGTCTGCATGGACCT3’, reverse: 5’CCA CGATGGTGACTTTGGCT3’, 18S (Housekeeping gene) forward: 5’CGGAGGTTCGAAGACGATCAGATA3’, reverse: 5’TTGG TTTCCC GGAAGCTGCC3’. Amplification program included 35 cycles of annealing at 61°C for 30 denaturation for 0.45 second at 95°C, annealing at 61°C for 30s and and final extension for one minute at 72°C. During thermal cycling, emission from each sample was recorded, and Rotor Gene Q software processed the raw fluorescence data to produce threshold cycle (Ct) value. The housekeeping gene 18S was used for internal normalization. The expression level was quantified by ΔCt value. ΔCt value was calculated by Ct value (target gene) - Ct value (housekeeping gene). The relative expression level or fold change was calculated by 2-ΔΔCt.
Statistical Analysis
Statistical analysis was carried out using standard statistical methods (SPSS software version 16.0). Data were expressed as the mean±SEM. Comparisons between the groups were made by student’s t-test (2-tailed) or one-way ANOVA with Tukey’s post-hoc analysis depending on number of groups. For all statistical tests, p< 0.05 was considered as significant.
Results
In the present study we treated cultured HUVEC with four OCPs at a concentration which are much below their toxic dose. These doses are based on the average amount of pesticide residue in human blood reported in various studies.
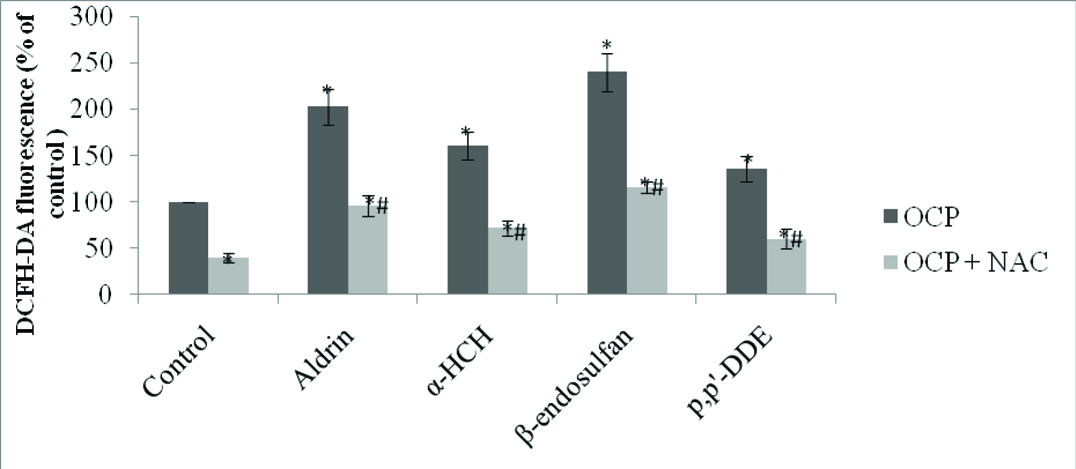
Intracellular ROS Level in Pesticide Treated Cells: To determine intracellular ROS generation by OCPs in HUVECs, we used DCFH-DA derived fluorescent signals as indicator of ROS production. HUVECs were treated with each of the four OCPs i.e., aldrin, β-endosulfan, α-HCH and p,p’-DDE at a concentration 0.1 μM for 24 hour and DCFH-DA fluorescence was measured. The results obtained are shown in [Table/Fig-1] and the data was presented as percentage of control. All the four pesticides induced significant increase in DCF fluorescence: a two fold increase with aldrin, a 1.6 fold increase with α-HCH, a 2.4 fold increase with β-endosulfan and a 1.3 fold increase with p,p’-DDE. Pre-treatment of NAC (10 μM) significantly inhibited (40-60%) ROS production in all the four pesticides treated HUVEC including control.
Intracellular ROS generation in pesticides treated HUVEC.
HUVECs (10,000 cells) were treated with four OCPs namely aldrin, β-endosulfan, α-HCH and p,p’-DDE at a concentration 0.1 μM for 24 hour, also pre-treated with NAC (10μM) with the same concentration and in control HUVEC treated with DMSO vehicle. The results are presented as mean±SEM of percentage of controls for at least three independent experiments. p<0.05 is considered as significant. * Significant vs control and # significant vs pesticide treated cells.
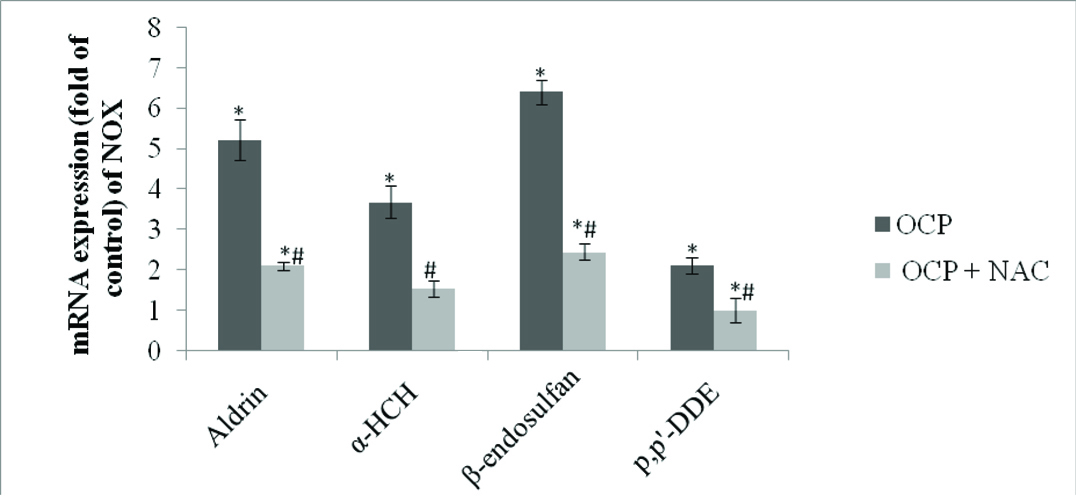
Effect of Pesticides on NOX Expression in HUVEC: To evaluate the source of ROS in pesticides treated HUVEC we determined mRNA expression of NOX cytosolic p47phox subunit of NOX2 by Real Time PCR. Significant increase in p47phox expression was observed in the HUVEC on treatment with OCPs. The results obtained are shown in [Table/Fig-2]. Of the four OCPs tested, maximum increase in NOX expression was observed in HUVECs treated with β-endosulfan (>6 fold) whereas least increase in expression was seen during p, p’-DDE exposure (~2fold). Pre-treatment of HUVEC with NAC significantly inhibited increased mRNA expression of NOX due to pesticides exposure ranging between 60-80%.
Expression of NOX in pesticides treated HUVEC.
Cells were treated with aldrin, β-endosulfan, α-HCH and p,p’-DDE at 0.1μM concentration for 24 hour and also pre-treated with NAC (10μM) with the same concentration. Results are expressed as mean±SE of three independent experiments. p<0.05 is considered as significant. * Significant vs control and # significant vspesticide treated cells.
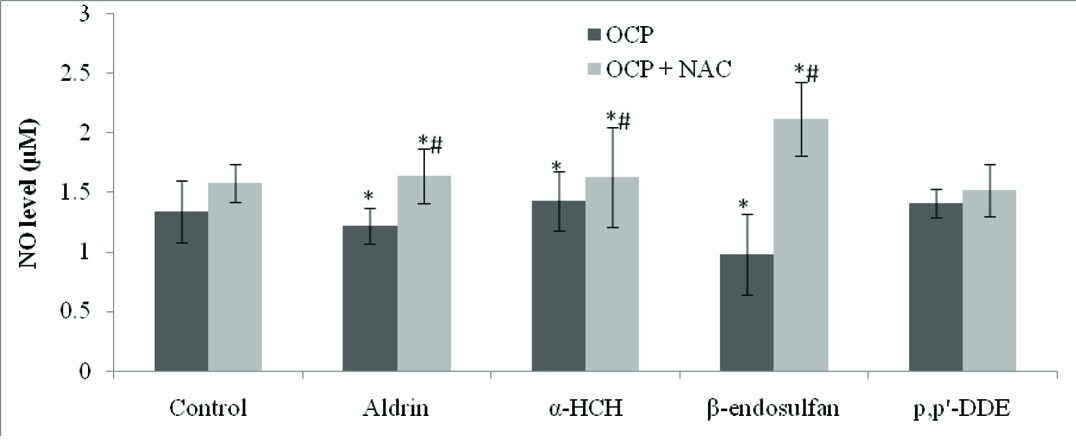
Effect of OCPs on NO Production in HUVEC: There was significant decrease in NO production in OCP-treated HUVEC as compared to controls [Table/Fig-3]. HUVEC treated with antioxidant like NAC showed significant increased amount in NO production as compared to OCP-treated HUVEC (p<0.01). The lowest level of NO was produced by HUVEC treated with β-endosulfan (p<0.01).
Effect of organochlorine pesticides on production of nitric oxide in HUVEC and ameliorating effect of NAC.
Bar chart showing the level of NO in control and different concentration of OCPs and the level of NO in NAC treated HUVEC and pre-treatment with NAC and different OCPs. Values are expressed in mean±SEM, *significant at p<0.01 as compared to controls, #significant at p<0.01 as compared to pesticide treated HUVEC.
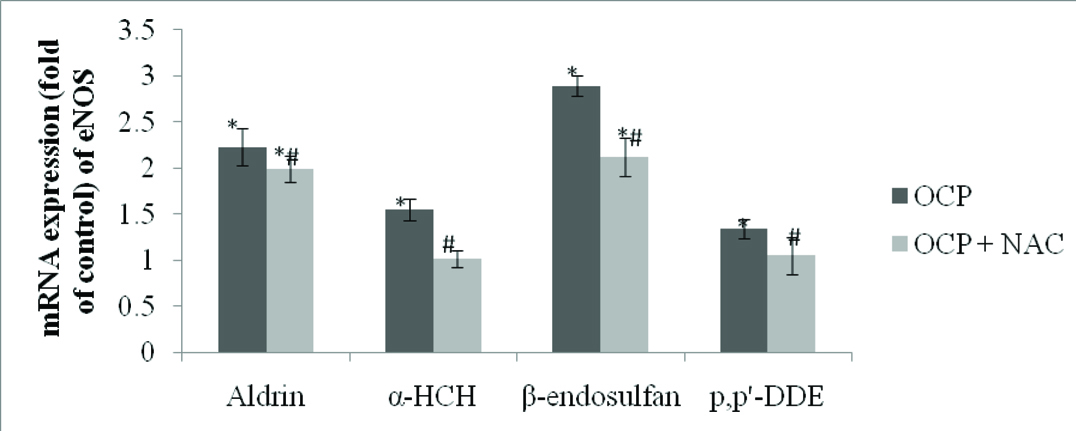
Effect of Pesticides on eNOS mRNA Expression in HUVEC: eNOS expression was estimated by real time PCR. All the tested pesticides exhibited significant up-regulation of eNOS at mRNA level [Table/Fig-4]. The maximum eNOS expression was observed with the exposure of β-endosulfan whereas p,p’-DDE exposure showed lowest effect on the eNOS mRNA expression. eNOS mRNA expression was maximally up-regulated to 2.23 fold on treatment of β-endosulfan, whereas least increase in expression was seen during p,p’-DDE exposure (1.34 fold). Expression of eNOS was significantly down-regulated with the pre-treatment with NAC.
Expression of eNOS in pesticides treated HUVEC.
Cells were treated with 0.1μM concentration of aldrin, β-endosulfan, α-HCH and p,p’-DDE for 24 hour and also pre-treated with NAC with the same concentration. Results are expressed as mean±SE. * indicates the significant difference from controls. # indicates the significant difference with respective OCPs.
Discussion
In the present study, the effects of OCPs were investigated in vitro for ROS generation and expression of NOX and eNOS enzyme system in endothelial cell. We focused on four OCPs because these compounds are detected in the blood of general Indian population which are shown to be associated with metabolic and CVD risk. The doses used for the study were 0.1 μM which is way below the toxic level of these pesticides and are based on their average presence in human blood samples extrapolated to cell culture.
It was observed that micro-molar concentration of the above-mentioned pesticides induced ROS generation in HUVEC. Earlier dieldrin and p,p’-DDE have been shown to generate elevated ROS levels in monocytes [14]. Also in a previous study Kannan K et al., have reported oxygen radical generation in Jurkat T cells treated with endosulfan [15]. DDT and its metabolites have been shown to induce ROS generation in PBMC [16]. Therefore, it is evident that these agrochemicals can induce ROS generation that is responsible for adverse biological effects.
NOX is believed to be the major source of ROS in endothelial cells [17]. We have found significantly increased expression of NOX by HUVEC when treated with the OCPs as compared to control. This increased expression of NOX clearly suggests that OCPs induced up-regulation of this enzyme and thereby causing increased generation of ROS. Earlier, p,p’-DDE was shown to activate NOX accompanied by reduction in antioxidant enzymes such as SOD and catalase along with reduced Glutathione (GSH) content [18].
Endothelial cell derived NO plays a crucial role in regulating a wide spectrum of function in the cardiovascular system. NO is synthesised by eNOS in the endothelial cell and endothelial eNOS/NO dysfunction have been shown to be associated with atherosclerosis and CVD [19]. Since, detectable blood levels of OCPs are shown to be associated with CVD, we examined whether these OCPs can cause imbalance in endothelial eNOS/NO system. The results obtained by us showed that NO availability in the endothelial cells decreased with exposure to OCPs. To determine the reduction in NO due to OCPs exposure we further examined the expression pattern of NO producing enzyme NOS. We determined mRNA expression profile of eNOS gene by real time PCR. The result obtained showed significant up-regulation of eNOS mRNA. It is not clearly understood that while there is up-regulation of eNOS, there is decrease in NO level. Endothelial NO level is determined by the balance between synthesis, utilization and degradation of the molecule. It is possible that ROS particularly superoxides react with NO forming peroxynitrite thereby decreasing the bioavailability of NO and leading to increased eNOS expression is an attempt by the cells to restore parity of NO. This view is supported by the fact that studies with atherosclerotic animal model have reported increase in eNOS expression in endothelial dysfunction. Similar kind of responses have also been reported in HUVEC showing increased expression of eNOS mRNA [20].
NAC is a derivative of the amino acid cysteine. It is known for its role as an anti-oxidant and has various uses in Medicine. There was decreased production of ROS in HUVEC exposed to OCPs when pre-treated with NAC as compared to treatment with OCPs alone. This observation suggests that oxidative stress due to increased ROS generation is responsible for endothelial dysfunction and NAC through scavenging of ROS reduced the effect of OCPs on HUVEC and increased the bioavailability of NO.
OCPs induce endothelial dysfunction through increased ROS generation via NOX expression and reduced bioavailability of NO. This finding may explain the association of OCPs with CVD and atherosclerosis.
Limitations
The present report assessed expression of NADPH oxidase and eNOS by measurement of respective mRNA by using real time PCR. Although mRNA expression is a quantitative method it is always good to know the protein level of the respective gene product by Western Blotting which could not be done due to technical reasons.
Conclusion
Organochlorine pesticides induce endothelial dysfunction through increased ROS generation via NADPH oxidase expression and reduced bioavailability of nitric oxide. This finding may explain the association of OCPs with CVD and atherosclerosis.
[1]. Poljsak B, Fink R, The protective role of antioxidants in the defense against ROS/RNS-mediated environmental pollutionOxid. Med. Cell. Longev 2014 2014:1-22. [Google Scholar]
[2]. Alavanja MCR, Ross MK, Bonner MR, Increased cancer burden among pesticide applicators and others due to pesticide exposureCA Cancer J Clin 2013 63:120-42. [Google Scholar]
[3]. Saxena RGP, Jain DK, In vitro anti-oxidant effect of vitamin E on oxidative stress induced due to pesticides in rat erythrocytesToxicol Int 2011 1:73-6. [Google Scholar]
[4]. El-Shenawy NS, Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytesToxicol in vitro 2010 4:1148-57. [Google Scholar]
[5]. Ilkhanipour JM, Heydari R, Farshid AA, Salehi S, The effects of vitamin E on endosulfan-induced oxidative stress in rat heartPak. J Nutr 2007 4:375-80. [Google Scholar]
[6]. Slaninova A, Smutna M, Modra H, Svobodova Z, A review: Oxidative stress in fish induced by pesticidesNeuroendocrinol Lett 2009 30:2-12. [Google Scholar]
[7]. Chen S, Meng XF, Zhang C, Role of NADPH oxidase-mediated reactive oxygen species in podocyte injuryBio Med Res Int 2013 2013:1-10. [Google Scholar]
[8]. Chalupsky K, Cai H, Endothelial dihydrofolatereductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthaseProc Natl Acad Sci USA 2005 102:9056-61. [Google Scholar]
[9]. Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, de Chantemele EB, Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidaseArterioscler Thromb Vasc Biol 2008 28:1627-33. [Google Scholar]
[10]. Lee DH, Lind PM, Jacobs DR, Salihovic S, van Bavel B, Lind L, Background exposure to persistent organic pollutants predicts stroke in the elderlyEnviron Int 2012 47:115-20. [Google Scholar]
[11]. Vichova T, Motovska Z, Oxidative stress: Predictive marker for coronary artery diseaseExp Clin Cardiol 2013 18:e88-e91. [Google Scholar]
[12]. Deo SH, Jenkins NT, Padilla J, Parrish AR, Fadel PJ, Norepinephrine increases NADPH oxidase derived superoxide in human peripheral blood mononuclear cells via αadrenergic receptorsAm J Physiol Regul Integr Comp Physiol 2013 10:R1124-132. [Google Scholar]
[13]. Forstermann U, Li H, Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncouplingBr J Pharmacol 2011 164:213-23. [Google Scholar]
[14]. Mangum LC, Borazjani A, Stokes JV, Matthews AT, Lee JH, Chambers JE, Organochlorine insecticides induce NADPH oxidase-dependent reactive oxygen species in human monocytic cells via phospholipase A2/arachidonic acidChem Res Toxicol 2015 4:570-84. [Google Scholar]
[15]. Kannan K, Jain SKM, Oxygen radical generation and endosulfan toxicity in Jurkat T-cellsCell Biochem 2003 247:1-7. [Google Scholar]
[16]. Perez-Maldonado IN, Herrera C, Batres LE, Gonzalez-Amaro R, Diaz-Barriga F, Yanez L, DDT-induced oxidative damage in human blood mononuclear cellsEnviron Res 2005 2:177-84. [Google Scholar]
[17]. Frey RS, Ushio-Fukai M, Malik AB, NADPH Oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiologyAntioxid Redox Signal 2009 4:791-810. [Google Scholar]
[18]. Song L, Liu J, Jin X, Li Z, Zhao M, Liu W, p, p’-Dichlorodiphenyldichloroethylene induces colorectal adenocarcinoma cell proliferation through oxidative stressPLos One 2014 11:1-12. [Google Scholar]
[19]. Yang Z, Recent advances in understanding endothelialdysfunction in atherosclerosisClin Med Res 2005 4:53-65. [Google Scholar]
[20]. Wu XL, Du LZ, Xu XF, Relationship between expression of endothelial nitric oxide synthase and NADPH oxidase in lungs of mice exposed to chronic hypoxiaEur PMC Plus 2015 17:1001-06. [Google Scholar]