Introduction
IVM of oocytes is an effective method for the production of mature oocyte used in ART, which includes IVF cloning and Intra Cytoplasmic Sperm Injection (ICSI). IVM is a cost-benefit technique with few side effects for gonadotropin stimulation of IVF, and is a simple treatment for certain infertile couple [1–4]. The GV-stage oocytes which are stripped of cumulus cells have a reduced developmental capacity when compared with cumulus enclosed GV-stage oocytes [5,6]. Cumulus cells play an important role in oocyte maturation, since they provide and transfer several known and unknown factors that are essential in the regulation of meiotic progression, normal nuclear and cytoplasmic maturation of oocytes and subsequent embryonic development after fertilization [7,8]. Studies have demonstrated that inappropriate culture mediums increase ROS resulting in OS events, DNA damage and low quality of oocytes. These are the main factors that lead to failure in IVM and decrease productivity [9,10]. OS appears to be responsible for many injuries in the embryo and oocyte [9,11,12]. Embryo metabolism generates ROS via several enzymatic mechanisms that penetrate the cell membrane and pass through it to damage cellular molecules such as lipids, proteins, and nucleic acids [13]. BME and CYS are thiol compounds that stimulate glutathione (GSH) synthesis [14]. Despite the fact that there is more stability of CYS than other thiol compounds, the effect of this substance on the growth of oocytes or embryos depending on breed, dose rate and the medium, is shown differently.
GSH is one of the components of many biological processes, which includes the construction of DNA, protein metabolism of drugs and chemicals, and it protects the cells during OS events [15]. In addition, GSH and its revival mode of reproduction and early development of organism play a unique role. It can reduce the generation of free radicals such as hydrogen peroxide and oxygen free radicals, which are disruptive to oocytes. It has been shown that GSH plays an important role in oocyte maturation [15]. Intracellular GSH is an essential part in the development of the oocyte cytoplasm. Activities of GSH-related antioxidant properties protect the oocyte at the front of the active molecules of oxygen is toxic. With this consideration, this study was designed to evaluate the effects of antioxidants (BME and CYS) on improving ART invitro which is essential. Therefore, the main purpose of this study was to ascertain whether enriching the oocyte medium with antioxidants, BME and CYS may improve IVM and fertilization and embryo development into blastocyst, of mouse immature oocyte.
Materials and Methods
This experimental study was carried out in the cellular and molecular research center at Yasuj University of Medical Sciences in Iran. The University ethic committee approved the design of study. Sixteen mice were housed under standard laboratory conditions (temperature of 20±2°C, relative humidity of 40-45% and light-dark cycle of 12:12 hour) and had free access to standard laboratory food and water. Gonadotropin from pregnant mare serum, TCM 199, Fetal Bovine Serum (FBS), BME, CYS, sodium pyruvate, Epidermal Growth Factor (EGF), Follicle Stimulating Hormone (FSH), penicillin G, potassium and streptomycin sulfate salts, and Bovine Serum Albumin (BSA) were purchased from Sigma-Aldrich (Germany).
Collection of GV oocytes
The oocytes were obtained from 4-6 weeks old female NMRI mice [16]. Briefly, 10 IU of PMSG was IP injected into animals for ovarian stimulation. After 48 hours, the animals were immediately sacrificed by cervical dislocation and the ovaries were removed in TCM199 medium, supplemented with 10% FBS. GV oocytes were obtained by puncturing antral follicles with a 28-gauge needle under a stereomicroscope in a holding medium.
Invitro Maturation (IVM)
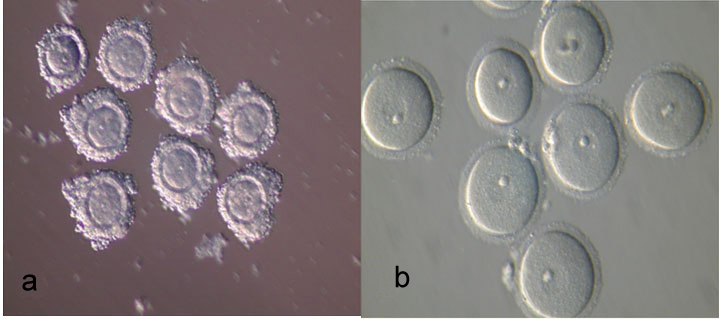
The GV oocytes collected were washed three times in the holding medium containing TCM199 supplemented with 10% FBS. GV oocytes were divided into two forms which include A: COC [Table/Fig-1a], and B: DO [Table/Fig-1b]. Each group was placed in 25 μl micro drops of maturation medium containing TCM199 supplemented with 10% FBS, 0.23 sodium pyruvate, 10 ng/ml EGF, 100 mlU FSH, 75 μg/ml penicillin G, potassium salt and 50 μg/ml streptomycin sulfate salt and incubated for 24 hours in a humidified atmosphere of 5% CO2 at 37°C. GV oocytes with cumulus or COC and without cumulus or DO cells were divided into four groups (experimental groups): Group 1: the COC cells cultured in TCM199 medium; Group 2: the COC cells cultured in medium containing CYS, BME and TCM199; Group 3: the DO cells cultured in TCM199 medium; Group 4: the DO cells cultured in medium containing CYS, BME and TCM199. After 24 hours incubation, the oocytes were observed with an inverted microscope and morphological changes in the nucleus or the extrusion of first polar body (MII) were used as criteria for nuclear maturation of GV-stage oocytes. Matured oocytes were collected for use in IVF.
Mouse oocytes with ×300 magnification by light-microscope. a: Cumulus oocyte complexes (COC); b: Denuded oocytes (DO).
Invitro fertilization (IVF)
Sperms were collected from epididymis of NMRI male mice aged 12 weeks. The sperm suspension (1×106 motile spermatozoa/ml) was capacitated for two hours in 1 ml T6 media supplemented with 15 mg/ml BSA. The invitro matured MII stage oocytes from each treatment group were placed into 0.9 ml T6 and 0.1 ml capacitated spermatozoa was added to it. After five hours incubation, the oocytes were washed through three droplets of T6 medium. Then, the oocytes were cultured in a droplet of T6 (25 μl) under mineral oil. They were assessed for cleavage to the 2-cells and blastocyst stage, respectively, after 24 and 120 hours.
Statistical Analysis
All the data were analysed with SPSS (version 15; USA) software using Chi-square test. The differences in the value of maturation, fertilization and development rates between groups were considered significant when p-value was less than 0.05.
Results
Maturation, fertilization and rate of development with availability of BME and CYS
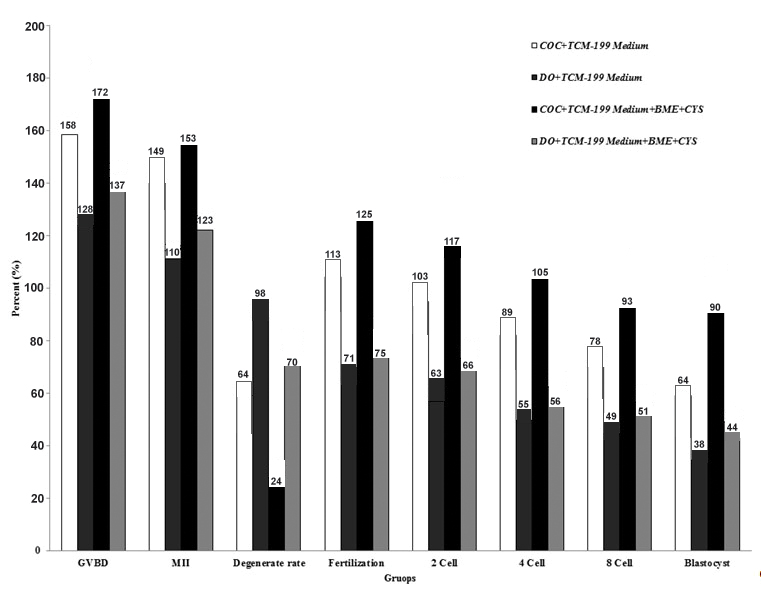
The main results of the descriptive data and study on the effect of adding BME and CYS on the maturity, fertilization and development rates of mouse oocytes are indicated in [Table/Fig-2]. A total number of 217 and 221 of GV stage oocytes for COC and DO, respectively, before addition of BME and CYS; and totally number of 173 and 180 of GV stage oocytes for COC and DO, respectively, after addition of BME and CYS, were included in this study. [Table/Fig-2] shows the evolution trend of the COC and DO from maturation to blastocyst stage in the control medium of TCM199 and the tested medium including TCM199+BME+CYS factors. So that total number of maturation (MII) was increased in TCM199 and COC as compared to the DO group (p-value=0.09). Furthermore, total number of maturation MII was statistically significant in TCM199 enriching with BME and CYS as compared to the DO group (p-value=0.03). It is demonstrated that COC significantly showed better trend as compared to oocytes without cumulus cover (DO), especially in the TCM199+BME+CYS medium.
Maturation, fertilization, cleavage rates and blastocyst development percentage differences of mouse GV oocytes in presence or absence of BME and CYS. COC: Cumulus Oocyte Complex; DO: Denuded Oocytes, M II: Maturation Rate, GVBD: Germinal Vesicle Breakdown
Formation of MII oocytes in the presence of BME and CYS
The change in GV stage oocytes to MII (maturation rate) in the treatment group with addition of BME and CYS (COC group) was statistically significant as compared to the DO group (p-value < 0.0001). Furthermore, the change in GV stage oocytes to MII in the treatment group without the presence of BME and CYS (COC group) was statistically significant as compared to the DO group (p-value=0.0013). [Table/Fig-3] shows the highest rate of maturation of oocytes cultured with cumulus in TCM199+BME+CYS medium and the lowest one in TCM199 medium without cumulus cells cultured without the addition of BME+CYS. It is also demonstrated that the maturation of the oocytes with cumulus cells was higher in all the groups during IVM in TCM199+BME+CYS medium when compared with the oocytes without cumulus cells in the TCM199 environment.
Frequency evolution of the oocytes with (COC) or without (DO) cumulus cover in TCM199 medium in availability of BME and CYS.* indicated significance differences in performing Chi-square test with p-value less than 0.05.
| Group | Total No. of GV oocyte examination | Total No. of GVBD (%) | Total No. of Maturation MII (%) |
|---|
| TCM199 | | | |
| COC | 217 | 158 (72.81) | 149 (68.66) |
| DO | 221 | 128 (57.91) | 110 (49.77) |
| TCM199+BME+CYS | | | |
| COC | 173 | 172 (99.42) | 153 (88.43) |
| DO | 180 | 137 (76.11) | 123 (68.33) |
Formation of 2PN (fertilization) in the presence or absence of BME and CYS
Fertilization rates or 2PN formation in the treatment group with the addition of BME and CYS and COC with cumulus was statistically significant as compared to the DO group without cumulus (p-value < 0.0001). In addition, the GV stage oocytes change to 2PN in the treatment group without the presence of BME and CYS (COC group) was statistically significant as compared to the DO group (p-value < 0.0001). As shown in [Table/Fig-4], maximum fertilization rates of oocytes with cumulus was observed in the TCM199+BME+CYS medium and the lowest one was in the group without cumulus with TCM199 medium in the absence of BME+CYS. Furthermore, fertilization of the oocytes with cumulus cells was higher in all the groups during IVF in TCM199+BME+CYS medium when compared with oocytes without cumulus cells cultured in TCM199 medium.
Comparing the evolution of the frequency distribution of oocytes with (COC) or without (DO) cumulus covering in presence of BME and CYS. A: before; and B: after treatment.
| Groups | Fertilization (%) | 2Cell (%) | 4Cell (%) | 8Cell (%) | Blastocyst (%) |
|---|
| Treatment 1 | | | | | |
| A (COC) | 113 (52.07) | 103 (47.46) | 89 (41.01) | 78 (35.94) | 64 (29.49) |
| B (COC) | 125 (56.56) | 117 (52.94) | 105 (47.51) | 93 (42.08) | 90 (40.72) |
| | | | | |
| Treatment 2 | | | | | |
| A (DO) | 71 (41.04) | 63 (36.41) | 55 (31.79) | 49 (28.32) | 38 (21.96) |
| B (DO) | 75 (41.66) | 66 (36.66) | 56 (31.11) | 51 (28.33) | 44 (24.44) |
Two-cell embryos and blastocyst formation in the presence of BME and CYS
Two-cell embryos formation or blastocyst development rate in the treatment group with addition of BME and CYS (COC group) was statistically significant as compared to the DO group (p-value < 0.0001). Furthermore, GV stage oocytes changes to two-cell embryos or blastocyst in the treatment group in the absence of BME and CYS (COC group) was statistically significant as compared to the DO group (p-value=0.0015; p-value= 0.0014, respectively). As indicated in [Table/Fig-4], the highest rate of 2 cell embryos or blastocyst was observed in the cumulus oocytes cultured in TCM199+BME+CYS medium and the lowest one was observed in the TCM199 medium without cumulus cover and cultured without the addition of BME+CYS. It is also indicated that 2-cell embryos or blastocyst formation in oocytes with cumulus cells was more than that in oocytes without cumulus cover. Furthermore, 2-cell embryos or blastocyst formation of the oocytes with cumulus cells (COC) was higher in all the groups in TCM199+BME+CYS medium (p-value=0.07), when compared with oocytes without cumulus cover (DO) cultured in TCM199 medium (p-value=0.36).
Discussion
The results of this study have shown that presence of cumulus cells enhances the oocyte maturation, fertilization rate and embryo development, but a lower percentage of oocytes without cumulus has reached these stages.
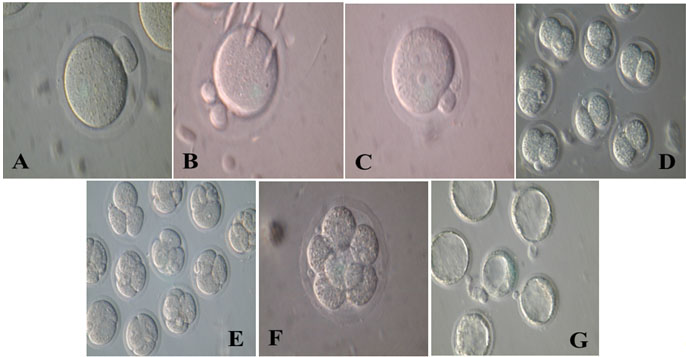
This result confirmed that cumulus cells induce meiosis and increase cytoplasmic maturation, while oocytes without cumulus cells have survived less due to the lack of coordination between the nuclear and cytoplasmic maturation [17–20]. It was suggested that cumulus cells may increase sperm fertilizing capacity, flexibility and induce changes in oocyte cytoplasm and zona pellucida, which can raise the possibility of normal fertility [21]. It appears that the cumulus cells increase 2-cells formation and blastocyst development rate [Table/Fig-5]. It is suggested that hormones and factors in follicular fluid exert their effects through the cumulus cells. Cumulus cells could enhance appropriate cytoplasmic maturation of oocytes which increase the ability of the embryo to develop after fertilization.
Evolution trend of the Cumulus Oocyte Complex (COC) and Denuded Oocytes (DO) maturation inTCM199 medium. A: Metaphase II (MII); B: Second polar body (after fertilization); C: Fertilization (2PN); D: Two-cell embryos; E: Four-cell embryos; F: Eight-cell embryos; G: Blastocyst.
The amount of blood flow and oxygen supply to the COC is dependent on the survival rate of embryos [22–26]. The results of this study showed that BME and CYS as antioxidants increased the development rate of bubble generator oocytes to GVBD and MII These results demonstrated that the application of 100 μM of BME and CYS in TCM199 culture medium showed the highest level of development on COC [27]. It indicated that adding 0, 10 and 100 μM of BME and CYS to the culture medium, increased the formation of embryo; and BME increased the number of cells in the produced cow embryos in the laboratory and free protein culture medium, which is consistent with the present findings [27].
In addition, the effect of 100 μM CYS on the evolution rate of mouse GV was investigated [28]. It was showed that CYS increased the level of oocyte development according to the result of a study; however, it was shown that CYS played no effective role in the number of embryos, which is contrary to the results of the present study. In this study, only one dose was used and the hybrid mouse was chosen, while in the present study, NMRI mice were used, which could be the reason for changes in the results of the two described study. In another study, the effect of 0, 100, 200 and 500 μM CYS on the development of embryo and its relation with the cumulus cell, the level of intracellular GSH and the development capacity of goat oocyte without cumulus complex was evaluated [29]. It was demonstrated that the presence of CYS and cumulus cells improved the GSH level and capacity development of goat IVM. This study showed that bare oocytes without cumulus cover (DO) cannot use CYS for GSH synthesis; otherwise, exogenous CYS can be added to the cumulus cells as a supplement [29].
The presence of 12.5 to 25 μM BME could increase GSH concentration in oocyte maturation and blastocyst development after fertilization in the laboratory; however, increase in the dosage of BME to more than 25-50 μM decreased the blastocyst development [30]. The results showed that certain concentrations of BME have advantageous effects on future embryo development and direct relationship with the level of intracellular GSH of porcine oocytes. The results are not the same with that of the present study. Difference in the results is due to types of animal examined, compounds in the culture medium and the presence of CYS in this study. These results indicated that high concentrations of BME considered for the types of animal could be toxic. Furthermore, embryo development after fertilization, matured pig oocytes in the presence of thiol compounds and various concentrations of CYS were studied. This study has shown the effect of different concentrations (0, 0.1, 0.2 and 0.4 mg/ml) of CYS in the presence of thiol compounds such as BME (25 μM) on maturation of oocytes of porcine and developed cumulus, nuclear maturity, level of internal GSH and subsequent embryo development after IVF [31,32]. It has been indicated that the presence of thiol compounds and adding CYS to IVM can increase GSH level and improve competitive environment of oocytes in fertilization process, which is in accordance with the present study, because both CYS and thiol compounds are used at the same time. In addition, it was demonstrated that supplementation of the medium with 0.6 mg/ml CYS during IVM and IVC could improve cow fetal development contrary to extracellular antioxidants such as catalase (CAT) and super oxide dismutase (SOD) [33].
Effect of 100 μM BME and/or 100 μM CYS on pig embryo formation rate was evaluated. The result indicated that the formed pronucleus levels in the various groups were not different [34]. But, cleavage rate was higher in CYS group used as compared to the BME group. This study has also shown that the rate of blastocyst formation was highest in the CYS group. These results are in accordance with the results of the present study; however, there are differences in the types of animals used [34]. Dose dependent influence of CYS on IVM and IVF and blastocyst development rates has been reported [35]. CYS could improve the organization of microtubules, the rate of GVBD and MII, which is in accordance with the present study results [35]. The results suggested that availability of BME and CYS in medium culture might have synergistic protective effect and could markedly improve IVM rate and evolution of COC to blastocyst.
Conclusion
In conclusion, selecting suitable environment for use in GV oocyte culture and revolution as progression method of ART invitro is important. Application of 100 μM BME and CYS is more effective in comparison with application of extracellular antioxidants such as CAT and SOD in protecting the GV oocytes from OS events, ROS and free radicals production which are the main factors resulting in unsuccessful IVM, and reducing reproduction or fertility. Oocyte with cumulus cells showed better response in comparison with non-cumulus cells in all the stages of this study treatment. Therefore, both environmental and cellular factors are important in ART. Because this protective effect is dose dependent, a suitable BME and CYS concentration should be chosen, and further human study needs to be designed. Furthermore, some clinical trials are suggested for this purpose.
[1]. Trounson A, Anderiesz C, Jones G, Maturation of human oocytes invitro and their developmental competenceReproduction 2001 121:51-75. [Google Scholar]
[2]. Hardy K, Wright CS, Franks S, Winston RM, Invitro maturation of oocytesBr Med Bull 2000 56:588-602. [Google Scholar]
[3]. Chian RC, Lim JH, Tan SL, State of the art in invitro oocyte maturationCurr Opin Obstet Gynecol 2004 16:211-19. [Google Scholar]
[4]. Mahmoudi R, Rajaei F, Kashani IR, Abbasi M, Amidi F, Sobhani A, The rate of blastocysts production following vitrification with stepwise equilibration of immature mouse oocytesIran J Reprod Med 2012 10:453-58. [Google Scholar]
[5]. Abbasi M, Akbari M, Amidi F, Kashani IR, Mahmoudi R, Sobhani A, Nitric oxide acts through different signaling pathways in maturation of cumulus cell enclosed mouse oocytesDARU 2009 17:49-52. [Google Scholar]
[6]. Nasiri E, Mahmoudi R, Bahadori MH, Amiri I, The effect of retinoic acid on invitro maturation and fertilization rate of mouse germinal vesicle stage oocytesCell J. (Yakhteh) 2011 19:19-24. [Google Scholar]
[7]. Mahmodi R, Abbasi M, Amiri I, Ragardi Kashani I, Pasbakhsh P, Saadipour K, Cumulus cell role on mouse germinal vesicle oocyte maturation, fertilization, and subsequent embryo development to blastocyst stage invitroYakhteh Med J 2009 11:299-302. [Google Scholar]
[8]. Khosravi-Farsani S, Sobhani A, Amidi F, Mahmoudi R, Mouse oocyte vitrification: the effects of two methods on maturing germinal vesicle breakdown oocytesJ assist reprod genet 2010 27:233-38. [Google Scholar]
[9]. Lian HY, Gao Y, Jiao GZ, Sun MJ, Wu XF, Wang TY, Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytesReproduction 2013 146:559-68. [Google Scholar]
[10]. Kiani-Esfahani A, Bahrami S, Tavalaee M, Deemeh MR, Mahjour AA, Nasr-Esfahani MH, Cytosolic and mitochondrial ROS: which one is associated with poor chromatin remodeling?Syst Biol Reprod Med 2013 59:352-59. [Google Scholar]
[11]. Zavareh S, Talebi A, Hasanzadeh H, Amending invitro culture condition to overcome oxidative stress in assisted reproduction techniques (ART)J Paramed Sci 2015 6:135-48. [Google Scholar]
[12]. Agarwal A, Gupta S, Sharma R, Oxidative stress and its implications in female infertility - a clinician’s perspectiveReprod Biomed Online 2005 11:641-50. [Google Scholar]
[13]. Khalil WA, Marei WF, Khalid M, Protective effects of antioxidants on linoleic acid-treated bovine oocytes during maturation and subsequent embryo developmentTheriogenology 2013 80:161-68. [Google Scholar]
[14]. de Matos DG, Gasparrini B, Pasqualini SR, Thompson JG, Effect of glutathione synthesis stimulation during invitro maturation of ovine oocytes on embryo development and intracellular peroxide contentTheriogenology 2002 57:1443-51. [Google Scholar]
[15]. Furnus CC, de Matos DG, Picco S, García PP, Inda AM, Mattioli G, Metabolic requirements associated with GSH synthesis during invitro maturation of cattle oocytesAnim Reprod Sci 2008 109:88-99. [Google Scholar]
[16]. Mahmoudi R, Subhani A, Pasbakhsh P, Abolhasani F, Amiri I, Salehnia M, The Effects of cumulus cells on invitro maturation of mouse germinal vesicle stage oocytesIran J Reprod Med 2005 3:74-78. [Google Scholar]
[17]. Akbartabar Toori M, Mosavi E, Nikseresht M, Jafari Barmak M, Mahmoudi R, Maturation influence of insulin-like growth factor-I on maturation and fertilization rate of immature oocyte and embryo development in NMRI Mouse with tcm199 and α-mem mediumJ Clin Diag Res 2014 8:AC05-AC08. [Google Scholar]
[18]. Mattioli M, Barboni B, Signal transduction mechanism for LH In the cumulus oocyte complexMol Cell Endocrinol 2000 161:19-23. [Google Scholar]
[19]. Mori T, Amano T, Shimizu H, Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured invitroBiol Reprod 2000 62:913-19. [Google Scholar]
[20]. Nikseresht M, Akbartabar Toori M, Rasti T, Ragerdi Kashani I, Mahmoudi R, The nuclear maturation and embryo development of mice germinal vesicle oocytes with and without cumulus cell after vitrificationJ Clin Diag Res 2015 9:AF01-AF04. [Google Scholar]
[21]. Vanderhyden BC, Armstrong DT, Role of cumulus cells and serum on the invitro maturation, fertilization and subsequent development of rat oocytesBiol. Reprod 1989 40:720-28. [Google Scholar]
[22]. Armstrong DG, Baxter G, Hogg CO, Woad K, Insulin-like growth factor (IGF) system in the oocyte and somatic cells of bovine preantral folliclesReproduction 2002 123:789-97. [Google Scholar]
[23]. Wandji SA, Wood TL, Crawford j, Levison SW, Hammond JM, Expression of mouse ovarian insulin growth factor system components during follicular development and atresiaEndocrinology 1998 139:5205-14. [Google Scholar]
[24]. Zhu L, Ohan N, Agazie Y, Cummings C, Farah S, Liu XJ, Molecular cloning and characterization of Xenopus insulin-1 growth factor-1 receptorEndocrinology 1998 139:949-54. [Google Scholar]
[25]. Zhou J, Chin E, Bondy C, Cellular pattern of insulin-like growth factor-I (IGFI) and IGF-I receptor gene-expression in the developing and mature ovarian follicleEndocrinology 1991 129:3281-88. [Google Scholar]
[26]. Zuelke KA, Glutathione (GSH) concentrations vary with cell cycle in maturation hamster oocytes, zygotes, and pre-implantation stage embryosMol Reprod Dev 2003 64:106-12. [Google Scholar]
[27]. Camano JN, Promotion of development of bovine embryos produced invitro by addition of cysteine and β mercaptoethanol to a chemically defined culture systemDairy Sci 1997 81:369-74. [Google Scholar]
[28]. Chen N, Liow SL, Yip WY, Tan LG, Ng SC, Influence of cysteamine supplementation and culture in portable dry incubator on the invitro maturation, fertilization and subsequent development of mouse oocytesTheriogenology 2005 63:2300-10. [Google Scholar]
[29]. Zhou P, Wu YG, Li Q, Lan GC, Wang G, Gao D, The interactions between cysteamine, cystine and cumulus cells increase the intracellular glutathione level and developmental capacity of goat cumulus-denuded oocytesReproduction 2008 135:605-11. [Google Scholar]
[30]. Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN, Presence of β-mercaptoethanol can increase the glutathione content of pig oocytes matured invitro and the rate of blastocyst development after invitro fertilizationTheriogenology 1998 50:747-56. [Google Scholar]
[31]. Abeydeera LR, Wang WH, Cantley TC, Rieke A, Day BN, Coculture with follicular shell pieces can enhance the developmental competence of pig oocytes after invitro fertilization: relevance to intracellular glutathioneBiol Reprod 1998 58:213-18. [Google Scholar]
[32]. Abeydeera LR, Wang WH, Cantley TC, Prather RS, Day BN, Glutathione content and embryo development after invitro fertilization of pig oocytes matured in the presence of a thiol compound and various concentrations of cysteineZygote 1999 7:203-10. [Google Scholar]
[33]. Ali AA, Bilodeau JF, Sirard MA, Antioxidant requirements for bovine oocytes varies during invitro maturation, fertilization and developmentTheriogenology 2003 59:939-49. [Google Scholar]
[34]. Kobayashi M, Lee ES, Fukui Y, Cysteamine or β-mercaptoethanol added to a defined maturation medium improves blastocyst formation of porcine oocytes after intracytoplasmic sperm injectionTheriogenology 2006 65:1191-99. [Google Scholar]
[35]. Mohammadi Roushandeh A, Habibi Roudkenar M, The influence of meiotic spindle configuration by cysteamine during invitro maturation of mouse oocytesIran Biomed J 2009 13:73-78. [Google Scholar]