Head and Neck Cancer (HNC) is the sixth most common cancer worldwide with the incidence of 6,33,000 cases and death of 3,55,000 cases as the annual burden [1]. It constitutes about 30% of all cancers occurring in India and majority of the cases occur in males [2]. Majority of them present with loco-regionally advanced disease constituting about 80% cases [3]. Treatment of such advanced cases with external beam radiation alone causes poor result in terms of cure, loco-regional recurrence and survival. To overcome this, Concurrent Chemoradiation Therapy (CCRT) has become one of the important developments in the management of Loco-Regionally Advanced Head and Neck Squamous Cell Carcinomas (LAHNSCC). Previous data showed the benefit of adding Chemotherapy (CT) to loco-regional therapy/Radiotherapy (RT) for non-metastatic unresectable disease [4,5].
CCRT is used in three major tumour types such as: Head and neck squamous cell carcinoma (HNSCC), undifferentiated nasopharyngeal carcinoma, and cervical cancers. CCRT or surgery followed by postoperative RT is current treatment approach in LAHNSCC [6]. Cisplatin based regimens were established as the most effective regimens with single agent activity, synergistic interaction and nonoverlapping toxicity. The cisplatin dose and delivery schedules have ranged from higher dose [100 mg/m2] every three weeks for three cycles to a low dose (6 mg/m2) daily administration [7]. Theoretically, high dose CT help in preventing distant metastasis by eradicating occult micrometastasis whereas low dose daily or weekly CT has pure radio sensitizing effect. There is insufficient data to suggest which CT schedule is superior in terms of better disease control. In the present study, we compared two CCRT schedules with weekly versus three weekly cisplatinum used for LAHNSCC. The aim of the present study was to compare the acute and late toxicity along with efficacy of both the CCRT schedules in LAHNSCCs in terms of response rate as well as locoregional control, and disease status in both the arms.
Materials and Methods
The present comparative prospective study was conducted at Acharya Harihara Regional Cancer Centre, Cuttack during the period of Nov 2011 to Oct 2012. The study was approved by institutional ethics committee. Thirty eligible patients of locally advanced carcinoma of oropharynx, hypopharynx, larynx, and oral cavity satisfying the eligibility criteria were included in this study.
Inclusion Criteria for the study were: histopathologically proven advanced stage (T3-4, N0-3, M0) Squamous Cell Carcinoma of oral cavity, oropharynx, hypopharynx and larynx, age more than 18 years and less than 70 years, normal haematological and biochemical parameters, Karnofsky Performance score of 70 or above, no history of prior Chemotherapy or Radiotherapy, and those who were willing to provide written informed consent. Exclusion Criteria for the study were: evidence of distant metastases by clinical or radiological examination, recurrent disease, prior Radiotherapy or Chemotherapy to Head and Neck, widely disseminated diseases, synchronous double primary malignancies, pregnant women, and simultaneous participation in another clinical study.
Pretreatment evaluations were: Complete history, including history of smoking, general and local examination, evaluation in Head and Neck Oncology, including endoscopic examination, complete haematological and biochemical profile, X-ray chest P-A view, ultrasound of abdomen and pelvis (if necessary), Radiological assessment of the disease extension by CT scan, or MRI, and histopathological study.
After pretreatment evaluation, patients were sequentially randomised into two arms: 15 patients were taken in each arm A and B [Table/Fig-1]. In both the arms, Radiotherapy was delivered to a dose of 66 Gy in conventional fractionation schedule with spinal cord sparing after 44 Gy by telecobalt machine.
Showing the study design.
EBRT = External beam radiotherapy
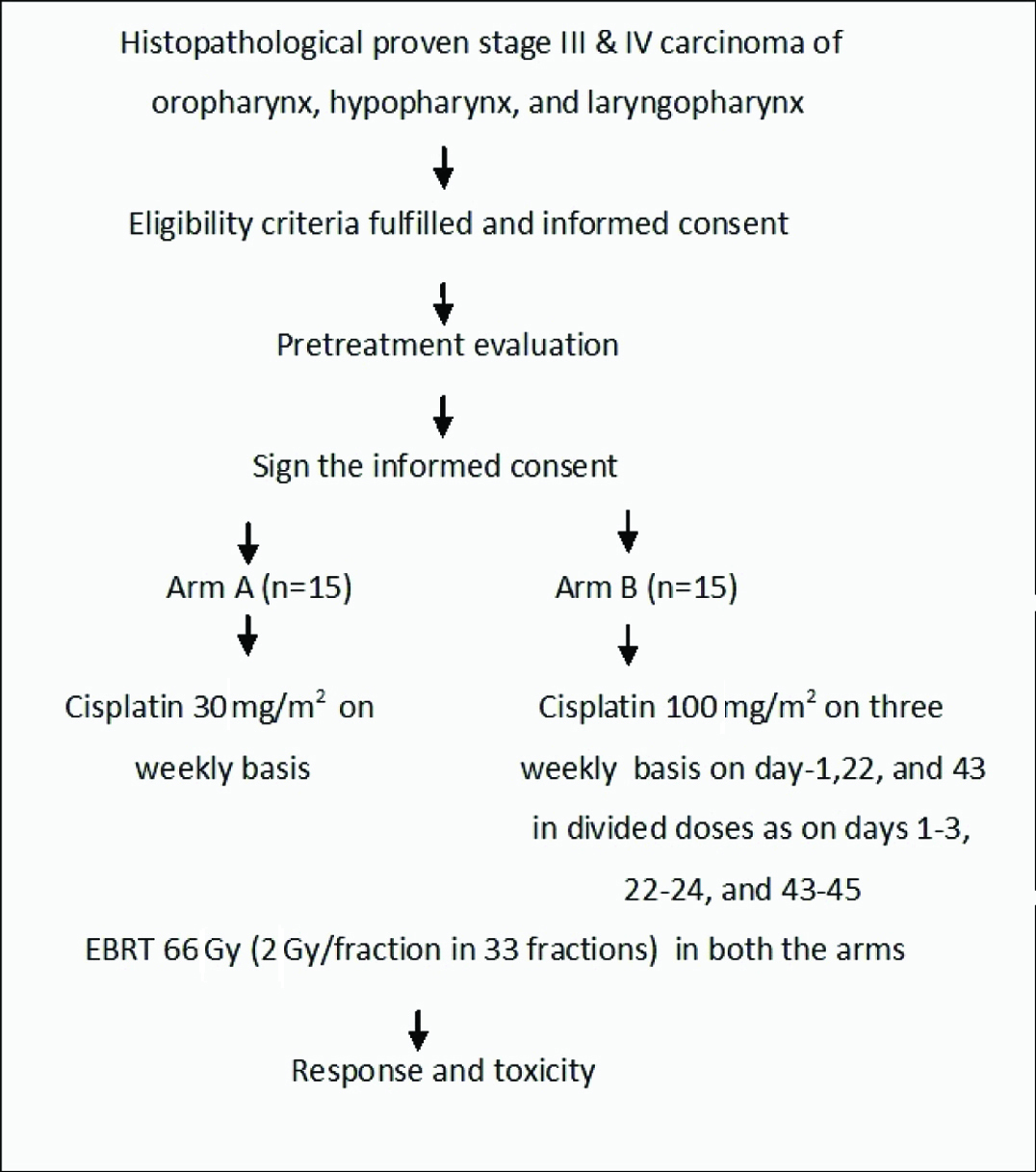
The primary endpoints of the study were disease response and toxicity profile. Patients were monitored on weekly basis during treatment for toxicity and nutritional support. After completion of treatment, patients were assessed at six weeks, three months, and seven months interval for response evaluation and toxicity. Toxicities were assessed according to Radiation Therapy Oncology Group Acute Radiation Morbidity Criteria [8].
Statistical Analysis
It was done by using Chi-Square test, as appropriate.
Results
Demographic data [Table/Fig-2]: The present study showed a prevalence of male gender, age group 45-65 years (27/30), well differentiated histology grade, oropharyngeal primary, and stage III disease [6,9–12].
Patient characteristics in comparison to literatures [6,9–12].
| Characteristics | Mitra D et al., (n=90) | Kang MH et al., (n=35) | Lu HJ et al., (n=117) | Fayette J et al., (n=262) | Rawat S et al., (n=60) | Our study (n=30) |
|---|
| Age (year): | Median: 52 | Median: 65 | Majority <65 (101; 86.3%) | Median: Fifth decade | Mean: Fifth decade | Median: 57.6 |
| Gender: M | 82(91.9%) | 35(100%) | 112(95.7%) | 227(86.64%) | 57(95%) | 27(90%) |
| F | 8(8.1%) | 0(0%) | 5(4.3%) | 0(0%) | 3(5%) | 3(10%) |
| Site: Oropharynx HypopharynxLarynxOral cavity | Larynx and hypopharynx > oral cavity and oropharynx | 15(42.9%) | 19(16.2%) | 125(47.70%) | 32(53.33%) | 16(53.34%) |
| 14(40%) | 41(35%) | 44(16.80%) | 3(5%) | 9(30%) |
| 3(8.6%) | 7(6%) | 51(19.48%) | 10(16.67%) | 4(13.33%) |
| 3(8.6%) | 47(40.2%) | 37(14.12%) | 15(25%) | 1(3.33%) |
| Stage: III | 52(57.78%) | NA | 14(12%) | 62(46.67%) | 19(31.67%) | 17(56.67%) |
| IV | 38(42.22%) | 35(100%) | 91(%) | 177(53.33%) | 41(68.33%) | 13(43.33%) |
| Histology grading: | | | | | | |
| Well differentiated | 39(43.33%) | 8(22.9%) | 61(52.1%) | NA | 10(16.67%) | 15(50%) |
| Mod. differentiated | 26(28.89%) | 21(60%) | 48(41.0%) | | 38(63.3%) | 13(43.33%) |
| Poorly differentiated | 25(27.78%) | 5(14.3%) | 8(6.8%) | | 12(20%) | 2(6.67%)) |
M = Male, F = Female, NA = Not applicable
CT/RT compliance: A 66.67% (10/15) patients completed six cycles of CT in weekly cisplatin arm, whereas, only 46.67% (7/15) patients were able to complete three cycles of three weekly cisplatin in arm B. A total of 13 patients (86.67%) completed 66 Gy RT in weekly arm, whereas, 12 patients (80%) in three weekly arm completed 66 Gy RT.
Toxicities [Table/Fig-3]: All the patients in both arms developed mucositis. However, few patients did not develop some toxicities in both the arms (Arm A: One patient without dysphagia, one patient without vomiting, four patients without anaemia, and two patients without leucopenia; whereas, Arm B: One patient without dermatitis, five patients without anaemia, and four patients without leucopenia). Grade-III mucositis and grade-III vomiting were more in three weekly arm in comparison to weekly arm (p-value: 0.729 and 0.360 respectively), whereas, grade-III dermatitis was more in weekly arm in comparison to three weekly arm (p-value: 0.360), though not statistically significant. Grade-III dysphagia/anaemia/leukopenias were almost equal in both the arms. There was almost no difference in late toxicities in both the arms (xerostomia and skin fibrosis).
Acute toxicities in patients in Arm A and B.
| Toxicities | Grade I | Grade II | Grade III | Grade IV |
|---|
| Arm A | Arm B | Arm A | Arm B | Arm A | Arm B | Arm A | Arm B |
|---|
| Acute | Mucositis | 1(6.67%) | 1(20%) | 8(53.33%) | 5(33.33%) | 6((40%) | 8(53.33%) | 0 | 1(6.67%) |
| Dermatitis | 2(13.33%) | 5(33.33%) | 9(60%) | 8(53.33%) | 4(26.67%) | 1(6.67%) | 0 | 0 |
| Dysphagia | 2(13.33%) | 4(26.67%) | 12(80%) | 10(66.66%) | 0 | 1(6.67%) | 0 | 0 |
| Vomiting | 4(26.67%) | 5(33.33%) | 9(60%) | 7(46.67%) | 1(6.67%) | 3(20%) | 0 | 0 |
| Anaemia | 3(20%) | 6(40%) | 7(46.67%) | 3(20%) | 1(6.67%) | 1(6.67%) | 0 | 0 |
| Leucopenia | 5(33.33%) | 6(40%) | 6(40%) | 4(26.67%) | 2(13.33%) | 1(6.67%) | 0 | 0 |
Response assessment: One patient in three weekly arm did not come for follow up. Response rate at three months after the completion of treatment, and median follow up of seven months were equal for both the arms. Overall, the complete response rate after a median follow up of seven months was 79.52%. Complete response was achieved by 73.33% (11/15) and 85.71% (12/14) of the patients in weekly and three weekly arms respectively but with no statistically significant difference.
Discussion
Previously, a variety of chemotherapeutic agents have been used with CCRT schedules either as monotherapy or combination therapy in the management of LAHNSCC with improved response rates such as: cisplatin, fluorouracil, methotrexate, bleomycin and mitomycin [9]. However, according to Pignon JP et al., cisplatin based CCRT offers the best survival advantages with 8% improvement in overall survival [5].
CCRT with 100 mg/m2 cisplatin is the standard of care for LAHNSCC in both adjuvant setting [13] and as definitive treatment [4,6]. However, severe grade III and IV toxicities in this schedule are the limitation for treatment protocol. According to Brizel DM et al., only 60% of patients were able to complete the three weekly cisplatin schedule [14]. According to Gupta T et al., Homma A et al., and Uygun K et al., weekly cisplatin based CCRT schedule have a similar response rate to the three weekly schedule suggesting weekly regimen could be of benefit in patients who are less likely to complete the standard three weekly schedule [15–17]. A cumulative dose of 200-250 mg/m2 cisplatin is required for a better locoregional control and survival benefit and can be achieved by splitting the three weekly cisplatin into a weekly cisplatin 30-40 mg/m2 schedule which might decrease toxicities and increase the compliance [14]. Also, weekly cisplatin can be administered on an outpatient basis and will reduce the cost of treatment by reducing hospital stay and supportive care. However, study comparison between these two CCRT schedules is limited and which schedule is superior in terms of side effects, locoregional control and survival is not clear. An alternative schedule with modified low-dose cisplatin (30-40 mg/m2) on weekly basis is the area of concern.
HNSCCs are common in the fifth and sixth decade of life and majority cases occur in males [6,9–12] and the present study supports most of data. In the present study, there was a predominance of oropharyngeal carcinoma, well differentiated histological grading, and stage III disease.
Treatment compliance: Duration of treatment has significant relation with treatment outcome. The duration of RT treatment time is a predictor of survival in LAHNSCC patients receiving CT and RT. In our study, 86.67% (13/15) patients in weekly cisplatin arm and 80% (12/15) patients in three weekly arm completed 66 Gy RT and most of the cases completed RT within 45-55 days. A total of 76.9% (10/13) patients in arm A and 66.67% (8/12) patients in arm B completed RT at eight weeks. A total of 23.1% (3/13) patients in arm A and 33.33% (4/12) patients in arm B completed RT at nine weeks. In our study 66.67% (10/15) of patients from weekly arm and 46.67% (7/15) in three weekly arm completed the planned course of chemotherapy cycles. According to Mitra D et al., the average delay in completing RT was 4.6 and 5.2 days for weekly and three weekly arms respectively [9]. According to Fayette J et al., completion of planned CT was 42.2% in three weekly arm and 66.7% in weekly arm. Temporary discontinuation of RT for toxicity, temporary arrest of RT ≥ 3 days, arrest of CT during RT were more in three weekly arm. Secondary hospitalization and RT interruptions (≥3 days) were found to be more but with a significantly better five year Overall survival (OS) (62.3% vs 52.6%) in three weekly arm than weekly arm [11]. According to Gupta T et al., patients receiving >85% of planned course of weekly cisplatin had a significantly superior 5-year local control (64.5% vs 41.8%), locoregional control (54.5% vs 26.8%) and Disease free survival (DFS) (49.6% vs 25.8%) [15]. According to Cooper JS et al., 61%, 23%, and 13% patients had received three cycles, two cycles, and one cycle of planned three weekly cisplatin based CCRT respectively [13]. According to Lu HJ et al., 94.9% of patients completed RT schedule and 75.2% of patients completed ≥ 6 cycles of weekly cisplatin [10]. According to Rawat S et al., 5/30 patients in weekly cisplatin arm and 10/30 patients in three weekly cisplatin arm had RT interruption due to toxicity, whereas, 10% patients in weekly arm and 20.7% patients in three weekly arm did not complete full course of CT (p = 0.15) [12]. According to Ho KF et al., there were more delays (41% vs 29%) in RT completion and more omissions (17.4% vs 5.6%) of CT in three weekly arm in comparison to weekly arm [18]. Some studies showed that a substantial fraction of patients were not able to receive the third planned dose of cisplatin suggesting a cumulative dose of 200 mg/m2 might be adequate to yield same beneficial effect [3]. However, according to Geeta SN et al., treatment interruption was significantly higher (41% vs 22%; p=0.005) in weekly arm [19].
Response assessment: In 1987, the Radiation Therapy Oncology Group (RTOG) first reported results from a phase II trial on CCRT using high dose cisplatin (100 mg/m2 on every three weekly basis). In our study, the complete response in weekly and three weekly cisplatin arms were 73.33% and 85.71% respectively with a trend towards increased complete response in 3-weekly arm in comparison to weekly arm although statistically not significant. A study by Rawat S et al. showed no difference in complete and partial response between weekly and three weekly arms [12]. Few retrospective studies showed a similar response rate to the three weekly cisplatin based CCRT schedule [15–17]. According to Mitra D et al., complete response in three weekly and weekly arms was 76% and 67% respectively [9]. Accordng to Homma A et al., the complete response rate was 98.1% in weekly cisplatin 40 mg/m2 based CCRT for stage II-IV HNSCC [16].
Toxicity assessment: In LAHNSCC, high grade adverse events reported for three weekly cisplatin schedule ranged from 77% to 85% [4,13]. The completion rate of this three weekly schedule is relatively low in CCRT arm (63-85%) [4]. According to Espeli V et al., weekly cisplatin has less adverse effect in comparison to three weekly arm [20], whereas, Uygun K et al., Ho KF et al., and Kose F et al., found no difference in toxicity profile [17,18,21] [Table/Fig-4] and Tsan DL et al., found less toxicity in three weekly arm [22].
| Serial No. | Study et al | No. of patients | Weekly vs 3-weekly cisplatin / cis-diamminedichloridoplatinum dose | Mucositis | Dermatitis | Vomiting | Neutropenia | Treatment compliance |
|---|
| 1 | Geeta SN et al. [19] | 83 | 40 vs 100 | ↑ in weekly | ↑ in weekly | - | ↑ in weekly | ↑ in 3-weekly |
| 2 | Ho KF et al. [18] | 51 | 33-40 vs 80-100 | No evidence | - | - | similar | ↑ in 3-weekly |
| 3 | Kose F et al. [21] | 55 | 30 vs 100 | ↑ in weekly | - | - | No evidence | No evidence |
| 4 | Mitra D et al [9] | 90 | 30 vs 100 | Similar But, grade III mucositis ↑ in weekly | Similar But grade III dermatitis ↑ in weekly | ↑ in weekly | ↑ in 3-weekly | Similar |
| 5 | Azony AE et al. [23] | 40 | 30 vs 100 | Similar | - | ↑ in 3-weekly | ↑ in 3-weekly | No evidence |
| 6 | Rawat S et al. [12] | 60 | 35 vs 100 | ↑ in 3-weekly | - | ↑ in 3-weekly | ↑ in 3-weekly | ↑ in weekly |
| 7 | Present study | 30 | 30 vs 100 | ↑ in 3-weekly | ↑ in weekly | ↑ in 3-weekly | Similar | ↑ in weekly |
Mucositis is the commonest in-field toxicity in CCRT. In our study, grade-III mucositis were 40% in weekly cisplatin arm in comparison to three weekly cisplatin arm where it was 53.33%, which was supported by Mitra D et al., (33.33% vs 40%), Fayette J et al., (grade-III/IV mucositis: 12.1% vs 34%; p<0.001), and Rawat S et al., (70% vs 75.9%; p=0.20) [9,11,12]. However, some studies showed higher grade-III mucositis in weekly arm in comparison to three weekly arm. In Azony AE et al., grade–III mucositis in three weekly arm and weekly arms were 5% and 10% respectively [23]. In a comparative study, severe acute toxicity (skin, haematological, treatment interruptions, weight loss, mucositis) was significantly higher (p=0.005) in the weekly cisplatin arm in comparison to three weekly cisplatin arm [19].
In our study, grade-II/III dermatitis in arm A was 86.67% and in arm B was 60%, whereas, according to Mitra D et al., it was 83.34% in weekly cisplatin arm and 86.66% in three weekly arm [9]. In our study, grade-II/III vomiting was equal in both the arm i.e. 66.67% (10/15), whereas, according to Mitra D et al., it was 86.67% in weekly arm and 80% in three weekly arm [9]. According to Gupta T et al., grade III emesis in weekly cisplatin (30 mg/m2) was 3.4% [15]. According to Azony AE et al., 5% of cases in weekly arm and 15% cases in three weekly arm developed grade III vomiting [23]. According to Rawat et al., 34.5% cases in three weekly arm and 20% cases in weekly arm developed vomiting [12].
When compared grade-III haematological toxicity in our study, it was 6.67% (1/15) for anaemia and 6.67% (1/15) for leukopenia in three-weekly arm, where as it was 61% in Cooper JS et al., [13]. According to Gupta T et al, grade III leucopenia was 5.7% [15]. In weekly arm, grade-III toxicity for anaemia was 6.67% (1/15) and for leucopenia it was 13.33% (2/15) in our study. According to Mitra D et al., 33% of cases in weekly arm and 43% cases in three weekly arm were with grade III neutropenia [9]. According to Rawat S et al., 55.2% cases in three weekly arm and 26.7% cases in weekly arm were with neutropenia [12]. According to Kang MH et al., Grade- III neutropenia, anaemia, stomatitis, and dermatitis were seen in 5.7%, 5.7%, 22.9%, and 82.9% cases respectively [6]. According to Fayette J et al., Grade III/IV -mucositis, -dermatitis, and -nauseas/vomiting in 3-weekly arm were 34%, 7.2%, and 4.1% respectively, whereas, in weekly arm were 12.1%, 1.2%, and 2.4% respectively [11].
There is not much difference in late morbidity between the two arms. A small study by Denis F et al. showed 47% of grade III/IV late toxicities were seen in RT alone whereas 82% in the CT-RT group [24]. According to Kang MH et al., grade-II xerostomia, dysphagia, and neck fibrosis were seen in 71.4%, 57.1% and 20.0% cases respectively [6]. Only 5.7% cases were with grade III xerostomia and there no grade-III dysphagia or neck fibrosis. According to Mitra D et al., late toxicities were found equally in weekly and three weekly arms and all patients are alive without any serious complications [9].
Limitation
The small sample size and shorter duration of follow-up are the limitations of the present study, in order to draw significant toxicity comparison between the two arms and to know the survival difference.
Conclusion
The present study concluded that, the complete response rate were slightly better in three weekly cisplatin arm compared to weekly cisplatin arm and there is a trend towards increase in grade-III mucositis and grade-III vomiting in three weekly cisplatin arm compared to weekly cisplatin arm. Among the two chemoradiation schedules, the difference in response rates and toxicities was statistically not significant. There is no large trial validating till date the superiority of weekly cisplatin versus three weekly cisplatin regimens. However, in order to decrease the toxicity and increase the future quality of life, weekly cisplatin based CCRT should not be used outside the clinical trials. Further studies with large sample size and longer duration of follow up are necessary to draw a conclusive data.
M = Male, F = Female, NA = Not applicable