Oxidative stress and inflammation, aggravates and increases the risk of CAD and its associated conditions such as Type 2 Diabetes mellitus [1]. Various toxic molecules either from the external source or from the normal metabolism need to be detoxified in the body. Glutathione S –Transferase (GST) is an important enzyme which detoxifies the toxic molecules and hence lowers the oxidative stress. GST is present in cytoplasm and mitochondria of almost every cell. This enzyme belongs to a multigene family of isoenzymes that catalyze the conjugation of reduced Glutathione (GSH) to a variety of electrophilic compounds as the first step in a detoxification pathway [2]. It also detoxifies toxic metabolites produced within the cell and protects cells from oxidant injury. The redox status of glutathione is highly essential for various biological events and its homeostasis is critically regulated by GST activity. Any alteration in GSH metabolism and GST activity can affect various signaling events leading to development of different diseases [3,4]. Hence, for this reason, GST has been assumed to play an important role in various diseased conditions. Several authors tried to study the correlation of GST gene polymorphism and GST activity with various diseased conditions but the conclusions are variable. It has been shown that GSTT1/GSTM1 polymorphism was a risk factor for Type 1 Diabetes in Slovak children and adolescence [5]. Another study showed that GST activity was significantly raised in both colorectal cancer and adenomas as compared to normal colonic tissue [6] but another study reported low GST activity in colorectal cancer patients [7]. Elevated plasma GST activity has also been reported in patients with hepatocellular damage [8,9]. Other studies regarding Diabetes mellitus and metabolic disorders reported variable association of GST gene polymorphism with these diseases [10] but they have not mentioned data regarding GST activity in their studies. Chronic alcoholism is associated with increased formation of reactive oxygen species and raised GST activity was thought to be protective against alcohol intoxication [11]. GST has been reported to be a sensitive marker for the diagnosis of alcoholic liver disease [12]. All these existing reports show that GST is an important biochemical tool which could provide significant information regarding oxidative stress prevailing in several diseases.
However, very little data is available regarding the status of GST activity in Coronary artery disease. Oxidative stress plays a key role in pathogenesis of CAD. Level of oxidative stress can be assessed by Total Antioxidant Status (TAS). The total antioxidant status is an indicator of the antioxidant potential of individual antioxidant levels in cells and plasma and hence can be of higher predictive value in assessing the CAD risk. Both, increased and decreased TAS levels, have been reported in CAD patients [13–15]. Antioxidant enzymes and molecules in the body give cellular protection against oxidative damage. Our main interest was to investigate the status of GST activity in Coronary artery disease. Patients with and without Type 2 Diabetes mellitus.
Materials and Methods
Present study was a case control study carried out in the Department of Biotechnology Swami Satyanand College of Management and Technology Amritsar in association with the Department of Biochemistry Government Medical College and Hospital Amritsar during the period from January 2015 to February 2016. The study comprised of diagnosed cases of CAD (n=133) and 110 age and sex matched healthy subjects as controls. All the subjects were in the age range of 30-60 years. These subjects were healthy volunteers without established complications. CAD patients were recruited from the Medicine Department of a private hospital in Amritsar. CAD patients were segregated into two groups:
Group-1 comprised of CAD patients (n=69) with Type 2 Diabetes mellitus (Fasting blood glucose>126mg/dl).
Group-2 comprised of CAD patients (n=64) without Type 2 Diabetes mellitus.
These patients (group-1) were on oral hypoglycaemic drugs but they were not on any regular antioxidant supplements. The study was approved by the institute’s ethical committee and informed consent was taken from all the subjects. A performa was designed to record the necessary details of patients as well as of controls regarding age, gender, occupation, present/past history of any disease, smoking, alcohol status and medication.
Patients with following conditions were excluded from the present study: lung disease, liver disease, renal disease, thyroid disease, habit of smoking, patients on hypolipidemic drugs and acute infections, chronic alcoholism.
Materials and Methods
A 10 ml of venous blood sample was taken from the antecubital vein of the CAD patients as well as from the apparently healthy controls, under aseptic conditions. The blood sample was divided into two halves: one in EDTA vial and another in plain vial to obtain serum. All the subjects were screened for the following biochemical investigations.
Study parameters
Serum Glutathione S-transferase assay was done using colorimetric method. Serum glutathione S-transferase activity was based upon the GST-catalyzed reaction between GSH and the GST substrate, 1-chloro-2, 4-dinitrobenzene (CDNB). The GST-catalyzed formation of GS-DNB produced a dinitrophenyl thioether which was detected by spectrophotometer at 340nm. One unit of GST activity was defined as the amount of enzyme producing 1 μmol of GS-DNB conjugate/min under the conditions of the assay. Serum Total Antioxidant Status (TAS) was assayed by procuring commercially available kit form Randox India Pvt Ltd. Briefly, 2,2 Azino-di-3-ethylbenzthiazoline sulphonae (ABTS) incubated with peroxidase (metmyoglobin) and hydrogen peroxide produced the radical cation ABTS+. This has a relatively stable blue-green color, which was measured at 600nm. Antioxidant in the added sample caused suppression of this color production to a degree which was proportional to their concentration.
GSH estimation was done in haemolysate using colorimetric method. Serum Lipid oxidation product (MDA) was estimated by the method of Beuge and Aust. Serum Lipid Profile was estimated with commercially available kit obtained from Randox Pvt., Ltd.
Statistical Analysis
The data were analysed by SPSS 16.0 software. Data were expressed as mean±SD standard deviation. The difference between the groups and sub groups were interpreted using student (t) test. A p-value less than 0.05 were considered statistically significant.
Results
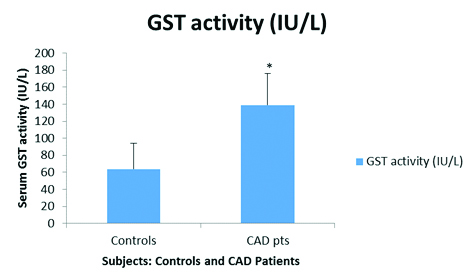
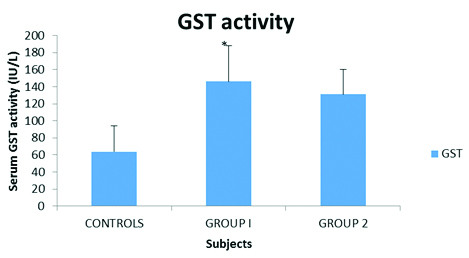
Serum GST activity was significantly raised (p<0.05) in CAD patients as compared to controls [Table/Fig-1]. Age and sex related variations in serum GST activity were insignificant (p>0.05; data not shown). As mentioned earlier, CAD patients were classified into two Groups: group-1 and group-2. Serum GST activity was significantly raised (p<0.05) in group-1 CAD patients i.e., with Type 2 Diabetes mellitus as compared to CAD patients in group-2 and controls [Table/Fig-2]. This clearly indicated the presence of marked oxidative stress in these patients. This was further supported from the observation that serum MDA levels were significantly high (p<0.05) in group-1 CAD patients [Table/Fig-3]. However, no significant variations (p>0.05) in plasma GSH levels were observed in this respect [Table/Fig-3].
Serum GST activity (IU/L) in CAD patients as compared to controls.
Control=64±30, CAD pts=139±37.3, *p<0.05.
Serum GST activity in CAD patients with and without Type 2 Diabetes mellitus in comparison to controls.
Control=64±30, Group 1=146.4±42.2, Group 2=131.2±29.3, *p<0.05.
Serum MDA and plasma GSH levels in CAD patients with and without type 2 diabetes mellitus in comparison to controls.
| Parameters | Controls n=110 | Group 1 n=69 | Group2 n=64 |
|---|
| MDA (μmol/l) | 5.9±6.4 | 8.2±8.0* | 7.2±5.4 |
| GSH (μmol/gm of Hb) | 8.8±2.5 | 8.8±4.7 | 8.3±5.3** |
*p<0.05 (significantly raised serum MDA levels in Group 1 patients as compare to controls and Group 2 patients), **p>0.05 (Insignificant difference)
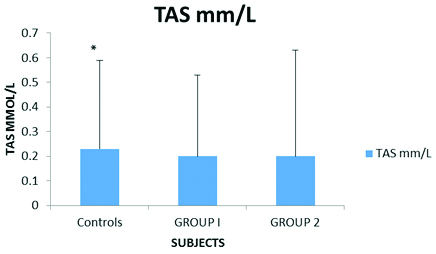
We were interested to evaluate the Total Antioxidant Status (TAS) of CAD patients with and without Type 2 Diabetes mellitus since plasma GSH levels did not show any significant variations. TAS was studied with the help of commercially available kit. TAS was highly depressed (p<0.05) in CAD patients (both group1 and 2) as compared to controls [Table/Fig-4].
Serum Total antioxidant status in CAD patients with and without type 2 Diabetes mellitus in comparison to controls.
Controls= 0.23±0.36, Group 1= 0.20±0.33, Group 2=0.20±0.43, *p<0.05
Serum lipid and lipoprotein cholesterol levels of patients and controls were also compared [Table/Fig-5] since this has been a routine investigation and needs to be checked. However, among all parameters, only serum triglyceride levels were observed to be significantly increased (p<0.05) in group-1 CAD patients as compared to controls. All other parameters did not show any significant variations (p>0.05).
Comparative account of serum lipid levels of CAD patients (Group 1 and Group 2) and controls.
| Parameters | Controls n=110 | Group 1 n=69 | Group2 n=64 |
|---|
| TC(mg/dl) | 148±46 | 155±47 | 160±42 |
| TG(mg/dl) | 127±48 | 166±96* | 154±84 |
| VLDL-C(mg/dl) | 33±52 | 32±20 | 39±63 |
| LDL-C(mg/dl) | 90±34 | 93±36 | 96±39 |
| HDL-C mg/dl) | 41±8.8 | 40±20 | 39±10 |
Group 1 CAD patients with Type 2 Diabetes mellitus
Group 2 CAD patients without Type 2 Diabetes mellitus
*p<0.05 Significantly high TG levels in Group 1 patients as compared to controls
Discussion
The role of oxidative stress has been established in coronary artery disease. GST is an important enzyme consisting of 3 supergene families; cystosolic, mitochondrial and membrane bound microsomal proteins which are involved in phase II detoxification of endogenous compounds and xenobiotics [16]. GST catalyzes the conjugation of reduced form of glutathione (GSH) to xenobiotics substrate for the purpose of detoxification [17].
We observed significantly increased serum GST activity in CAD patients as compared to controls, activity being more raised in CAD patients with Type 2 Diabetes mellitus. It could be possible that GST activity may be induced to combat the increased oxidative stress and hence can be speculated as a protective mechanism. GST works by conjugating GSH with several toxic molecules in order to detoxify them and facilitates their excretion. Hence, we were interested to evaluate the levels of GSH in this respect. However, insignificant difference was observed in plasma GSH levels between group-1 and group-2 CAD patients and controls. This indicates that it may be possible that GST activity and GSH do not bare any proportionate relationship and hence levels can vary. There was no significant correlation (p>0.05) observed between serum GST activity and plasma GSH levels. group-1 patients had an increased oxidative stress as reflected by the raised serum MDA levels, which is a by-product of auto-oxidation of polyunsaturated fatty acids [18].
Evaluation of Total Antioxidant Status (TAS) of CAD patients was highly important. TAS was assayed with commercially procured kit. Total antioxidant status was markedly low in CAD patients as compared to controls which further indicate increased oxidative stress in CAD patients. Other studies also reported low plasma TAS levels in patients suffering from coronary artery disease [14,15].
Oxidative stress is highly involved in the pathogenesis of CAD [19,20]. This information was not gained from GSH levels. Significantly increased serum GST activity in CAD patients especially with Type 2 Diabetes mellitus, increased MDA and low Total Antioxidant Status (TAS) is suggestive of increased oxidative stress prevailing with hyperglycaemia which could further enhance the process of atherosclerosis in CAD. Raised GST activity has been reported in Type 2 Diabetes patients as compared to healthy controls [21]. As far as the CAD is concerned, the existing studies mainly reported the association of GST polymorphism with coronary artery disease but did not mention the status of GST activity which may be affected by the GST polymorphism [22,23]. In the present study we evaluated GST activity. However, the GST polymorphism could give a better picture regarding its role in CAD and Type 2 Diabetes mellitus. Our results support the use of GST as an informative biochemical marker for the evaluation of oxidative stress in coronary artery disease and associated conditions like Type 2 Diabetes mellitus.
Limitation
We have not done the follow-up study which could be more informative evaluating the role of induced GST activity as a protective mechanism against oxidative stress. Moreover, evaluation of GST activity with GST gene polymorphism may provide additional information in CAD. We are planning to conduct study on GST gene polymorphism in our population.
Conclusion
Serum GST has been observed to be an informative marker of the prevailing oxidative stress in CAD patients with and without Type 2 Diabetes mellitus. Increased serum GST activity is a protective mechanism to combat increased oxidative stress.
*p<0.05 (significantly raised serum MDA levels in Group 1 patients as compare to controls and Group 2 patients), **p>0.05 (Insignificant difference)
Group 1 CAD patients with Type 2 Diabetes mellitus
Group 2 CAD patients without Type 2 Diabetes mellitus
*p<0.05 Significantly high TG levels in Group 1 patients as compared to controls