Acquired Immunodeficiency Syndrome (AIDS) caused by HIV is a major public health challenge of the modern era. While the entry of HIV in India was delayed, its spread has been very rapid since then and the disease has now reached epidemic proportions [1]. At present, India is home to the third largest population of HIV reactive patients in the world [2]. The progressive deterioration of the immune system by HIV makes an individual susceptible to a variety of opportunistic infections [3]. Nearly 80% of mortality in HIV positive patients is attributable to these infections rather than the virus itself [4]. Opportunistic pneumonias are considered to be a major contributor to this figure [5,6]. In addition, they are often the first clinical manifestation of an underlying HIV disease [7]. The spectrum of HIV-associated opportunistic pneumonias is quite wide and includes those caused by various bacterial, mycobacterial, fungal, viral and parasitic agents [6].
Fungal pneumonias, though an important bulk of opportunistic mycoses in HIV reactive patients, are often overlooked and underdiagnosed. The signs and symptoms are relatively non-specific and they are often masqueraded clinically and radiologically as pulmonary kochs [8,9]. A previous Indian study has estimated nearly 8.7% of HIV positive patients with lower respiratory tract infections to have a definite fungal aetiology [10]. The significance of fungi in causation of opportunistic pneumonias can be further highlighted by the fact that it was the opportunistic fungus Pneumocystis carinii that heralded the discovery of HIV/AIDS when reports of pneumonia caused by this agent in previously healthy men who had sex with other men and/or who were injection drug users first appeared in 1981 in the United States of America [11–13]. Pneumocystis carinii pneumonia (PCP) is included in the WHO list of AIDS indicative diseases [14]. While this pathogen continues to be the leading fungal aetiological agent causing opportunistic pneumonias in HIV infected, particularly in the western world, other fungi are also implicated including Cryptococcus neoformans, Aspergillus species and various endemic fungi such as Coccidioides immitis, Histoplasma capsulatum and Penicillium marneffei [6,14]. The incidence and aetiological spectrum of fungal opportunistic pneumonias varies according to the geographical region and continues to change with the evolving epidemiology of HIV infection [15].
Data from India on the prevalence and aetiology of HIV/AIDS-related fungal opportunistic pneumonias is limited and generally scarce. The present study was designed to elucidate the various fungal opportunistic pneumonias in HIV reactive patients seeking medical care at a tertiary care health facility in India, and to study the sociodemographic and CD4 profile of these patients.
Materials and Methods
The study population for the present prospective, cross-sectional analysis was a cohort of 234 symptomatic, confirmed HIV reactive patients attending a tertiary care health facility in Northern India. Participants were drawn from the wards and the Antiretroviral Treatment (ART) clinic of the health centre during a three year period spanning from October 2008 to September 2011. Inclusion criteria: i) confirmed HIV reactive patients ≥ 12 years of age with a clinical diagnosis of pneumonia (fever, productive cough, shortness of breath, chest pain, increased respiratory rate etc.); and ii) registered at the linked ART centre with CD4 counts performed at the time of enrolment. Exclusion criteria: i) Patients below the age of 12 years; and ii) registered patients referred from other ART clinics. Seventy one HIV seropositive patients including 52 males, 16 females and 3 transgenders of age 17-61 years were eligible for enrolment in the present study. HIV serostatus of the study participants was confirmed as reactive employing tests as per the algorithm described under strategy III of National AIDS control organization (NACO) guidelines [16].
At the time of enrolment, patients were evaluated using a standardized, structured predesigned questionnaire and a detailed history and clinical examination was performed. Information was gathered regarding the risk behavior profile of study subjects and an attempt was made in each case to elucidate the possible mode of HIV transmission. Following this, routine laboratory investigations and chest radiographs were obtained and CD4 counts done employing the FACS (Fluorescence-activated cell sorting) count system (Becton Dickinson, Singapore, BD). Written informed consent was obtained from each patient before recruiting them for the study and the study was duly approved by the Ethical Committee of the Institution.
Sputum samples were collected in sterile wide mouthed screw capped containers and transported to the laboratory as early as possible for further processing. Direct microscopy using Gram stain, 10% KOH wet mount and India ink preparations was performed. In addition, direct immunofluorescence for detection of P. carinii in sputum samples was done using MERIFLUOR-Pneumocystis kit (Meridian Bioscience).
Fungal culture was done for all sputum specimens employing SDA. For each sputum sample, cultures were streaked in duplicate and one set of inoculated media incubated at 25° and the other at 37°C. Culture tubes were examined every second day for growth up to six weeks before they were discarded as sterile. Any suspected fungal growth during this period was examined for gross colony morphology including colour, texture, topography and the underside or reverse. Further confirmation of the isolates was done by Gram staining, Riddles’s slide culture technique and lactophenol cotton blue preparation as per standard protocol [17]. Germ tube production, morphology on corn meal agar and carbohydrate fermentation and assimilation tests were employed for the identification and speciation of yeast isolates [17].
In addition, a venepuncture was performed in each patient following universal precautions and a 2-5 ml blood sample drawn and collected in a sterile centrifugation tube. Sera were separated by centrifugation and stored and preserved at-70°C till further tests were performed. The following battery of tests was put up for each serum sample: Cryptococcal Antigen Latex Agglutination System (CALASTM, Meridian Bioscience) for detection of cryptococcal capsular polysaccharide antigen; Platelia™ Aspergillus EIA (BioRad laboratories) for detection of Aspergillus galactomannan antigen; SERION ELISA antigen Candida (Institut Virion\Serion GmbH, Serion Immundiagnostica GmbH, Würzburg, Germany) for detection of Candida antigen and Histoplasma DxSelect™ (Focus Diagnostics) for detecting antibodies to Histoplasma species. The tests were performed as per the procedural details mentioned in the manufacturer’s guidelines.
Statistical Analysis
All positive results by microscopy and/or culture and/or serology were confirmed by subsequent sampling and repeat demonstration/isolation/testing. All the data was entered in Microsoft Excel sheet and the results analysed using the Epi info software, version 3.5.3, CDC, Atlanta, GA, USA. Descriptive statistics were shown in terms of proportions and figures; and arithmetic mean and standard deviation calculated for central tendencies and median for non-normal/skewed distributions.
Results
The mean age of HIV reactive patients enrolled in our study was 33.01±9.15 years (median=31 years; range=17-61 years). Most of the HIV reactive patients were in the age group of 21- 30 years (39.4%) followed by 31-40 years (32.4%). The number of males (52; 73.2%) was more than females (16; 22.5%) with a male to female ratio of 3.2:1. The study group also included three transgenders. The commonest mode of HIV transmission was heterosexual promiscuous which was documented in 58 (81.7%) of the study subjects. Other modes of HIV transmission recorded were homosexual in 3 (4.2%), intravenous drug abuse in 11 (15.5%) and blood transfusion in 4 (5.6%). The sociodemographic and CD4 profile of the study population is summarized in [Table/Fig-1].
Socio-demographic and CD4 profile of the study group (n=71).
| Variable | Number | Percentage |
|---|
| Age groups in years | ≤20 | 7 | 9.9% |
| 21-30 | 28 | 39.4% |
| 31-40 | 23 | 32.4% |
| 41-50 | 10 | 14.1% |
| ≥51 | 3 | 4.2% |
| Sex | Male | 52 | 73.2% |
| Female | 16 | 22.6% |
| Transgender | 3 | 4.2% |
| Marital status | Married | 57 | 80.3% |
| Unmarried | 14 | 19.7% |
| Residence | Rural | 15 | 21.1% |
| Urban | 56 | 78.9% |
| HIV* status of partner | Positive | 30 | 42.3% |
| Negative | 15 | 21.1% |
| Not tested | 26 | 36.6% |
| CD4 count (cells/μl) | 0-50 | 8 | 11.3% |
| 51-100 | 10 | 14.1% |
| 101-200 | 20 | 28.2% |
| 201-500 | 33 | 46.4% |
*Human immunodeficiency virus.
Employing immunofluorescence microscopy for detection of cysts and trophozoites of P. carinii, 14 (19.7%) of 71 patients were diagnosed with Pneumocystis Carinii Pneumonia (PCP) in our study. Eight of these patients had CD4 counts below 200 cells/μl, with a CD4 count as low as 16 cells/μl documented in one of them. In addition, ten of these 14 patients showed evidence of underlying active tuberculosis.
A positive direct microscopy and culture clinched the diagnosis of pulmonary aspergillosis in 7 (9.9%) patients. Sera of five of these patients were positive for galactomannan antigen and these were thus, diagnosed as proven cases of invasive pulmonary aspergillosis. The common Aspergillus species recovered were Aspergillus flavus in four, Aspergillus fumigatus in two and Aspergillus niger in one patient respectively. A predisposing lung condition in the form of pulmonary tuberculosis (TB) was identified in two; PCP in two and a TB-PCP co-infection in one of these seven patients respectively.
Six (8.5%) cases were diagnosed with candida pneumonia. Gram stained smear of sputum from all six cases revealed numerous budding yeast cells with pseudohyphae formation. In addition, sera of all these patients were positive for Candida antigen. The Candida species isolated included Candida albicans (C. albicans) in four, Candida glabrata (C. glabrata) in one and Candida tropicalis (C. tropicalis) in one of these six patients respectively.
Two (2.8%) patients were diagnosed with pulmonary cryptococcosis. While one of them had pulmonary involvement alone, the other presented with a concomitant cryptococcal meningitis. Images of the fungi identified in our study are provided as [Table/Fig-2,3,4,5,6,7 and 8].
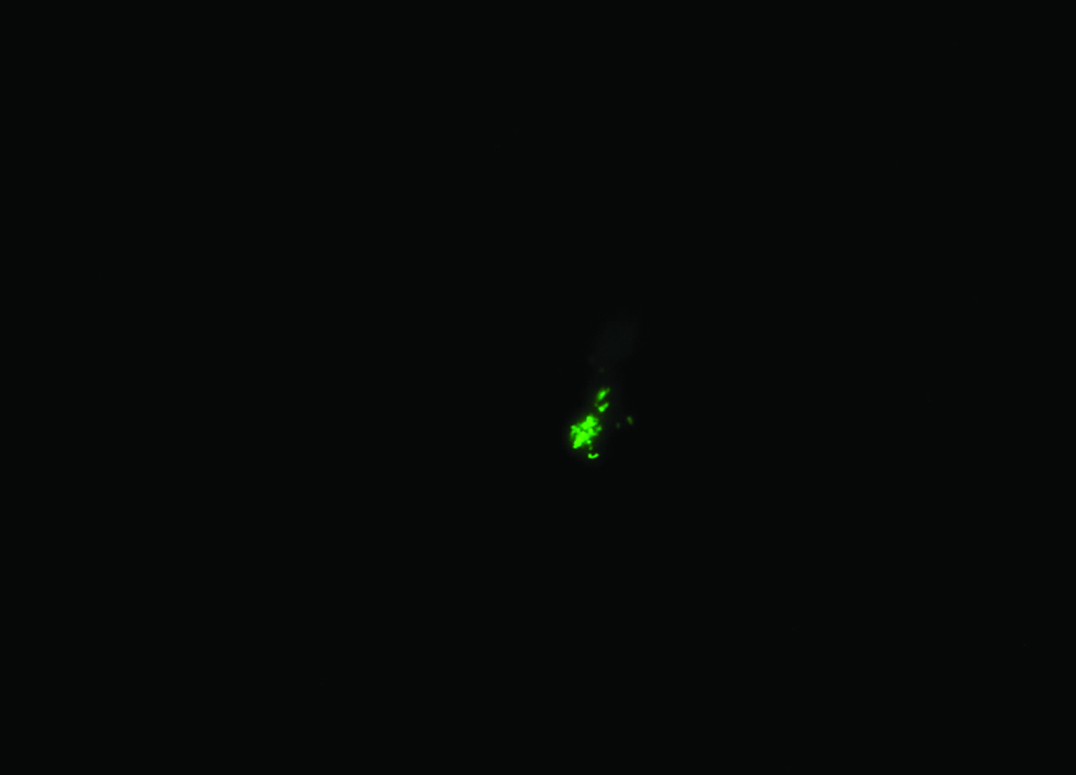
Direct immunofluorescence showing Pneumocystis carinii in a sputum sample.
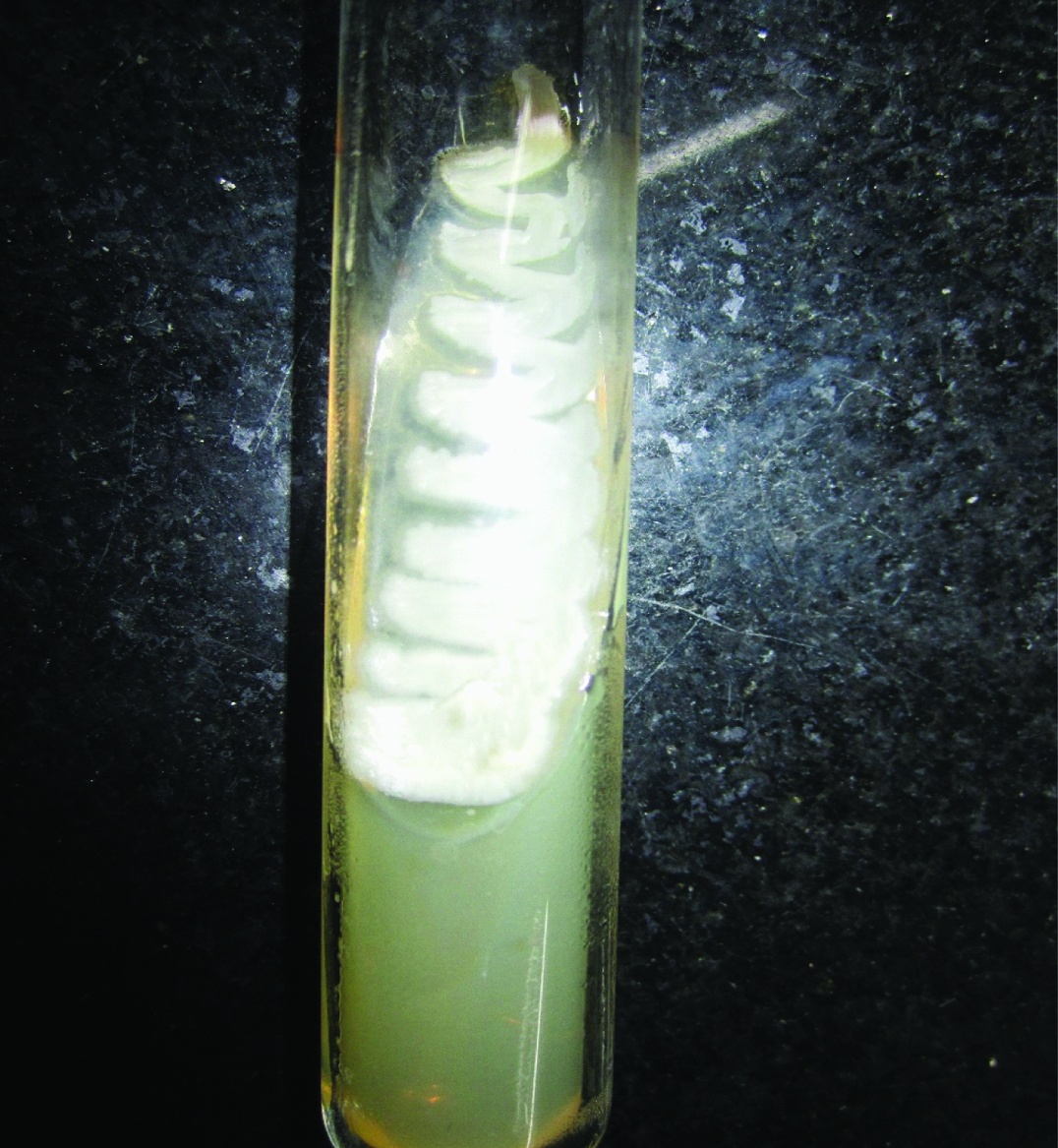
Growth of Aspergillus niger on Sabouraud Dextrose Agar.

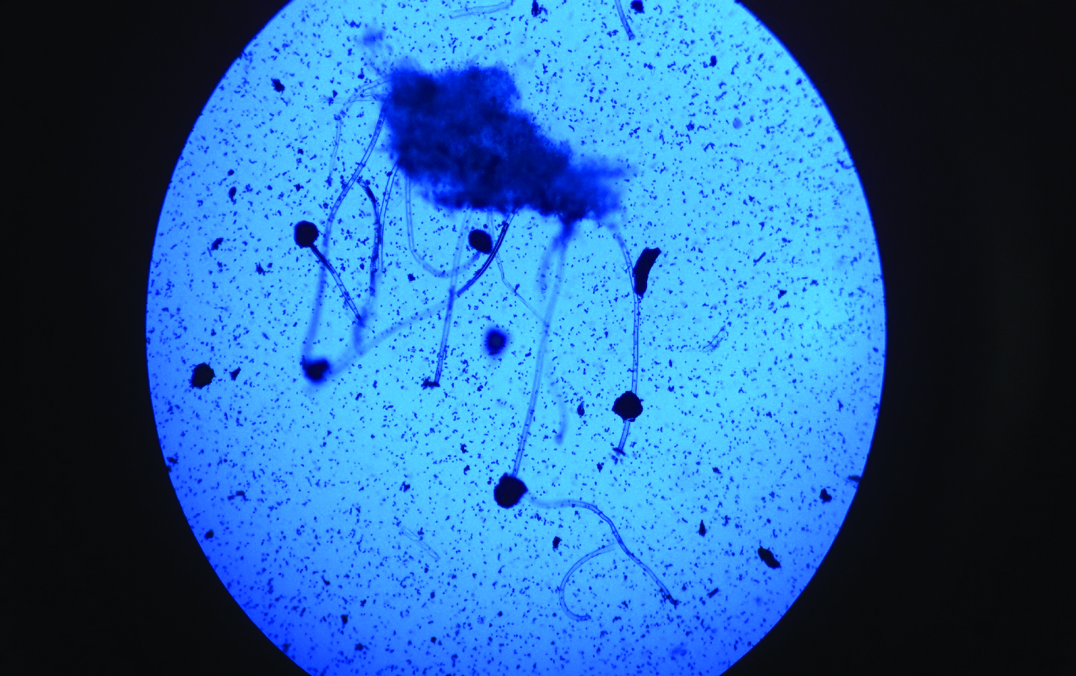
Microscopic morphology of Aspergillus niger (five-day-old culture on Sabouraud Dextrose Agar). Lactophenol cotton blue mount; magnification x 100.
Growth of Candida albicans on Sabouraud Dextrose Agar.
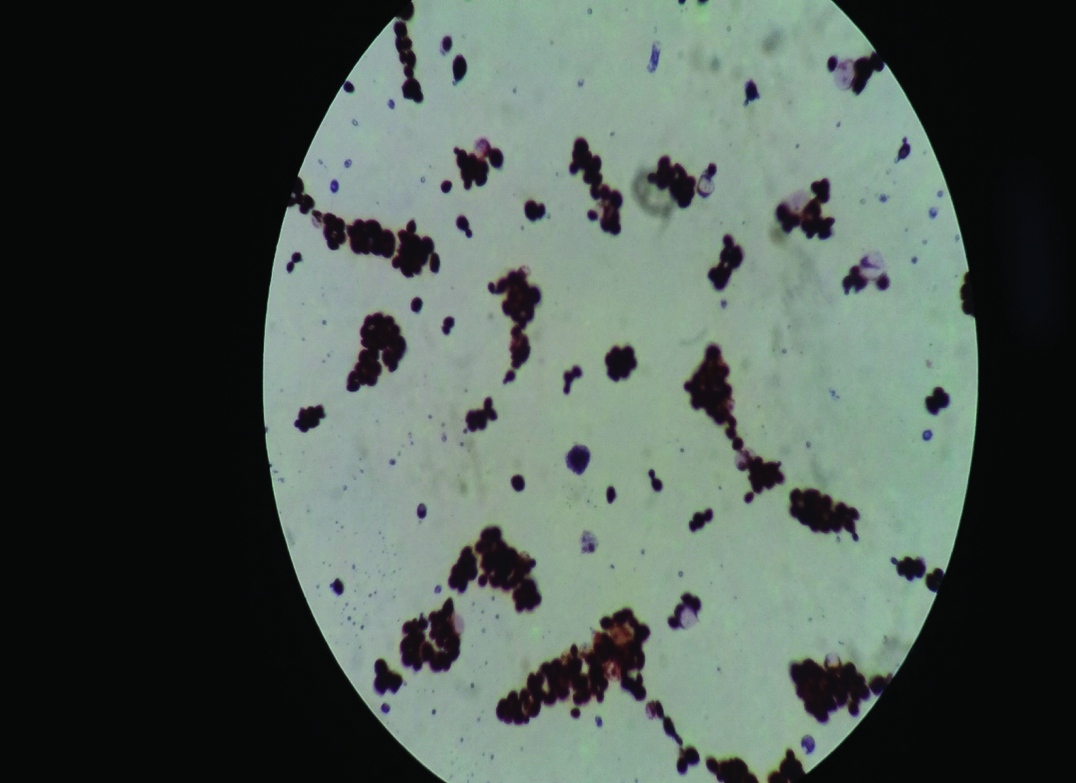
Microscopic morphology of Candida albicans (five-day-old culture on Sabouraud Dextrose Agar). Gram Stain; magnification x 1000.
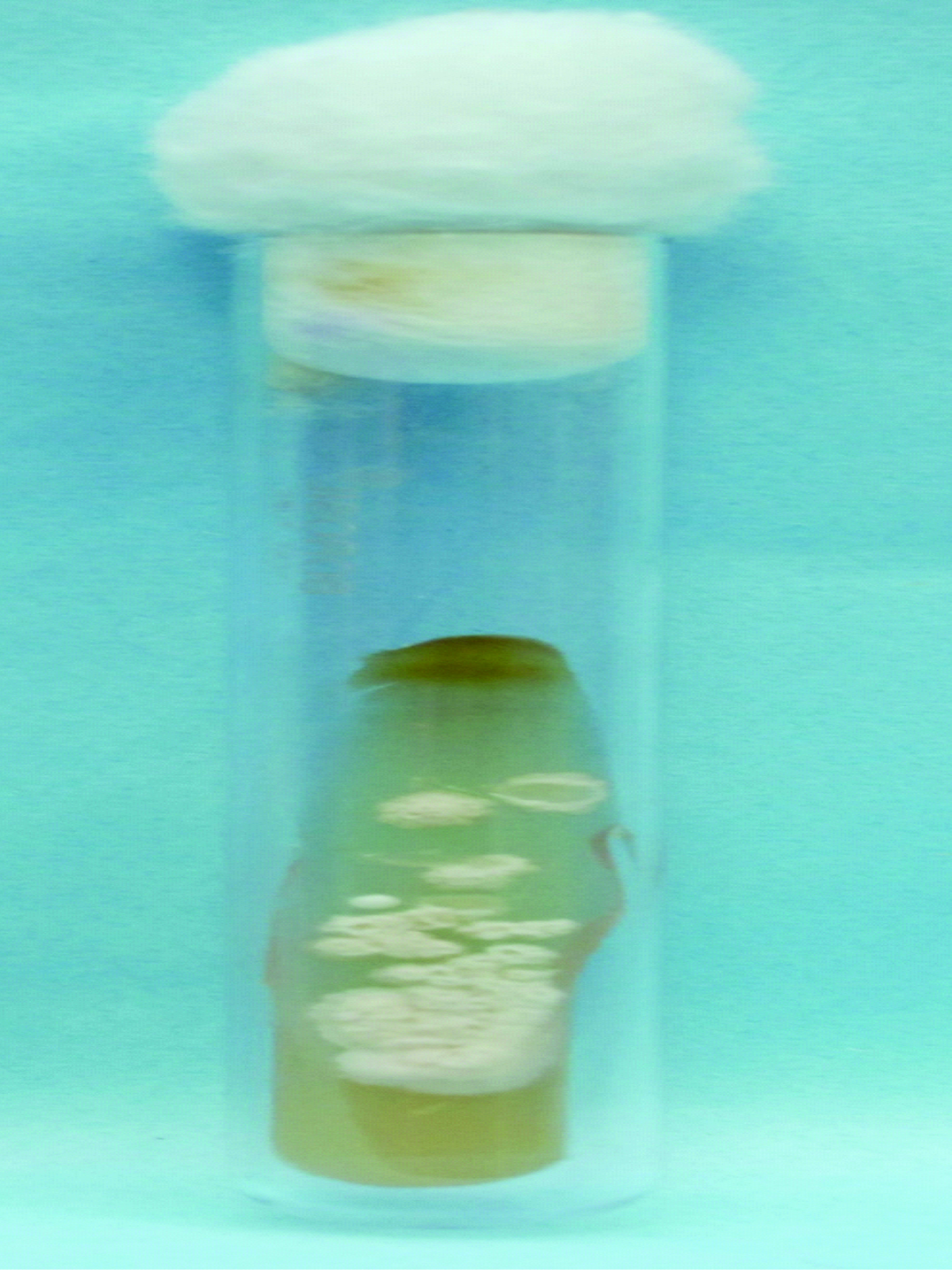
Growth of Cryptococcus neoformans on Sabouraud dextrose agar.
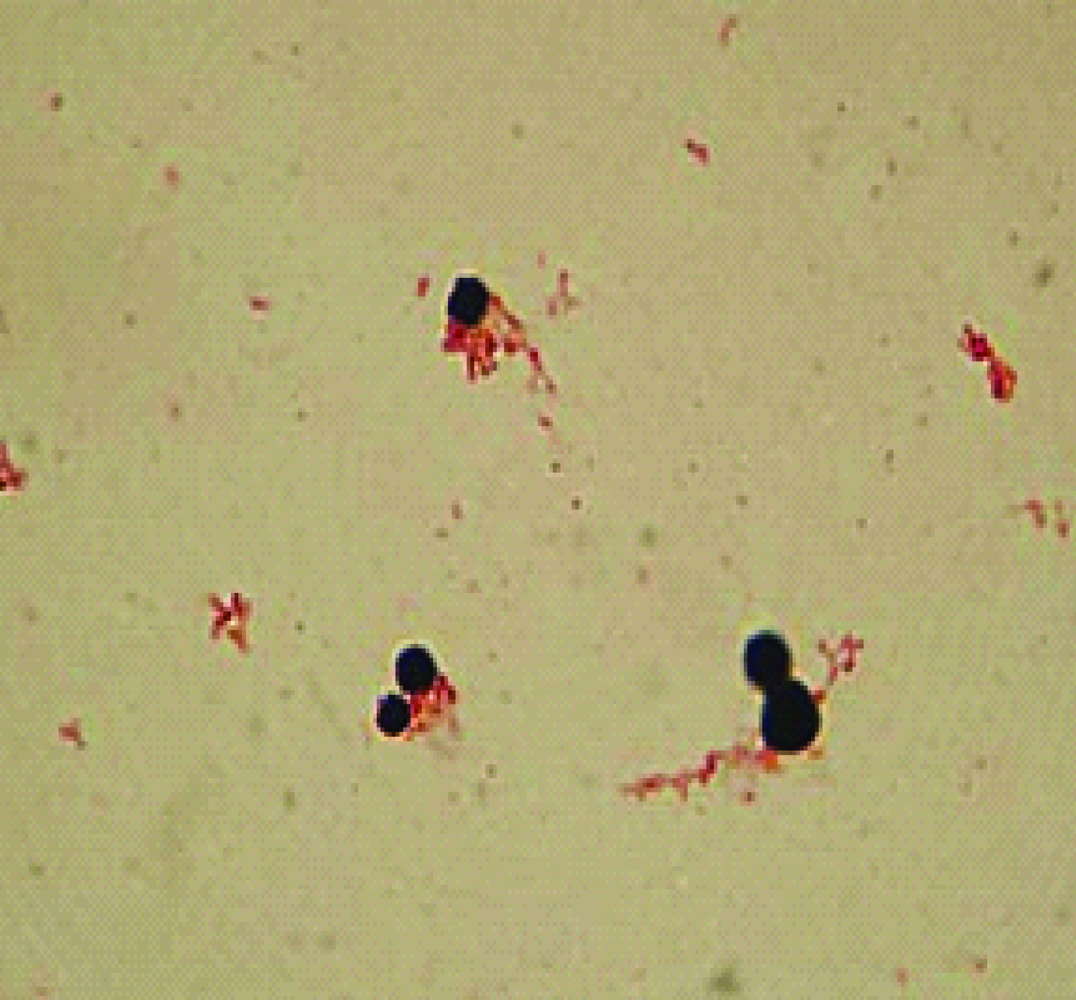
Microscopic morphology of Cryptococcus neoformans (five-day-old culture on Sabouraud Dextrose Agar). Gram Stain; magnification x 1000.
Serological evidence of a Histoplasma infection was seen in 21 (29.6%) cases. While in 15 of these patients Histoplasma was the sole fungal pathogen suspected in causation of pneumonia, the remaining six patients already had an established fungal aetiology.
Thus, of the total 71 HIV reactive patients with pneumonia, a definite fungal aetiology was established in 25 (35.2 %). Of these, sputa of 21 patients yielded single fungal isolates, while mixed fungal isolates were reported in four patients. Fever (21; 84%) followed by cough (15; 60%) was the most common presentation of fungal pneumonia. Further, 19 (76%) of the HIV reactive patients diagnosed with a fungal pneumonia had an underlying pulmonary tuberculosis. A summary of the sociodemographic, clinical and CD4 profile of these patients is provided in [Table/Fig-9].
Profile of HIV* positive patients with fungal pneumonia (n=25).
| Variable | Number | Percentage |
|---|
| Age groups in years | ≤20 | 2 | 8 % |
| 21-30 | 9 | 36 % |
| 31-40 | 9 | 36 % |
| 41-50 | 4 | 16 % |
| ≥51 | 1 | 4 % |
| Sex | Male | 20 | 80 % |
| Female | 4 | 16 % |
| Transgender | 1 | 4 % |
| Fever | Present | 21 | 84 % |
| Absent | 4 | 16 % |
| Cough | Present | 15 | 60 % |
| Absent | 10 | 40% |
| Mode of HIV transmission | Heterosexual contact | 22 | 88 % |
| Homosexual contact | 1 | 4 % |
| Blood transfusion | 1 | 4 % |
| Intravenous drug abuse | 1 | 4 % |
| Underlying TB† | Present | 19 | 76 % |
| Absent | 6 | 24 % |
| Chest radiograph findings | NAD‡ | 14 | 56 % |
| Infiltrates | 4 | 16 % |
| Pleural effusion | 6 | 24 % |
| Hyperinflation | 1 | 4 % |
| CD4 count(cells/μl) | 0-50 | 3 | 12 % |
| 51-100 | 4 | 16 % |
| 101-200 | 5 | 20 % |
| 201-500 | 13 | 52 % |
*Human immunodeficiency virus; † Tuberculosis; ‡ No abnormality detected.
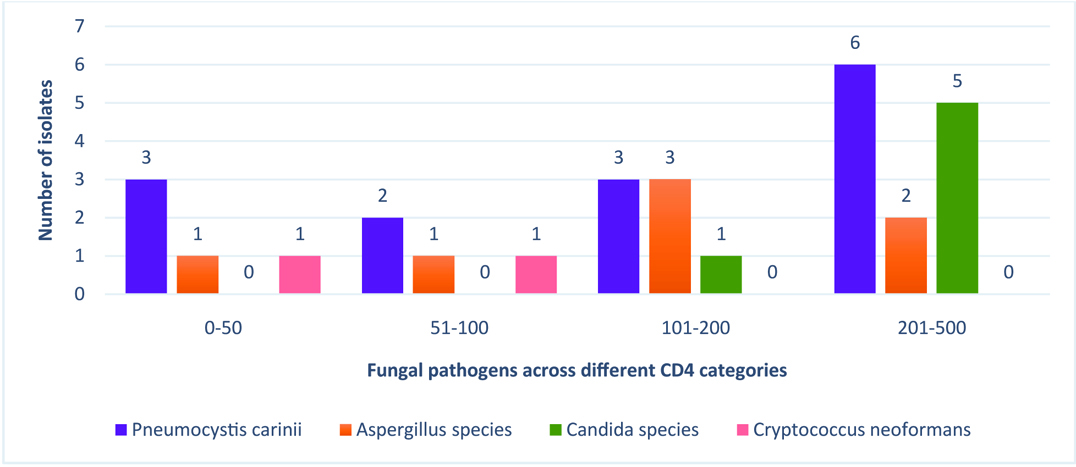
Patients with a cryptococcal pneumonia had the lowest CD4 counts (mean=66.00±26.87 cells/μl; median=66 cells/μl) when compared to those with pneumonia due to other fungal pathogens. In contrast, HIV positive patients with a positive sputum culture for Candida species were noted to have higher CD4 counts than those with other fungal pneumonias. The mean and median CD4 counts of patients with opportunistic pneumonia due to various fungal agents are summarized in [Table/Fig-10]. With regard to distribution of fungal pathogens across different CD4 categories, it was observed that cryptococcal pneumonia was exclusively diagnosed in HIV/AIDS patients with CD4 counts < 100 cells/μl, while Candida species were isolated in patient groups with CD4 counts 101-200 and 201-500 cells/μl. The occurrence of various fungal opportunistic pneumonias across different CD4 categories is depicted in [Table/Fig-11].
Opportunistic fungal pneumonias and CD4 counts of patients.
| Fungal pathogen | Number | Mean CD4 count(cells/μl) | Median CD4 count(cells/μl) | Range of CD4 count(cells/μl) |
|---|
| Pneumocystis carinii | 14 | 145.57±100.19 | 121.5 | 16-324 |
| Aspergillus species | 7 | 155.86±119.33 | 117 | 18-329 |
| Candida species | 6 | 269.33±86.47 | 299.5 | 120-355 |
| Cryptococcus neoformans | 2 | 66.00±26.87 | 66 | 47-85 |
Correlation of CD4 counts with fungal organisms isolated.
Discussion
HIV associated opportunistic pneumonias can have a rapid progression in the absence of prompt and appropriate treatment. A proper diagnostic evaluation is therefore, vital for effective patient management.
Fungi are generally non pathogenic in immunocompetent individuals [9]. However, with the emergence of HIV pandemic, fungal opportunistic infections are becoming common [18]. Most of the fungal infections are AIDS indicative illnesses and manifest at low CD4 counts [19]. Fungal opportunistic pneumonias are an important cause of mortality and morbidity among people living with HIV/AIDS [6]. The clinical signs and symptoms of fungal pneumonias are generally non specific. An accurate diagnosis is therefore, essential and calls for a combination of microbiological, serological and histopathological techniques. Early diagnosis is imperative for effective clinical management and for institution of appropriate preventive strategies so that the associated mortality and morbidity can be reduced.
We report PCP in 21.1% of HIV positive patients with pneumonic involvement. A similar figure has been reported by Mishra et al., who detected P. carinii in 25.34% of their patients [20]. On the contrary, a similar study from another tertiary care centre in north India documented PCP in only 1.87% of the study subjects [10]. Previous Indian data suggests, variable but higher figures [21–23]. Perhaps PCP in India has largely been under-reported and the reasons for this are manifold. In most of the cases, PCP was marked by co-existing and more prevalent and virulent AIDS related conditions such as TB to which the patient succumbed even before a diagnosis of PCP was made and on other occasions the lack of availability of sensitive and sophisticated diagnostic tools precluded a possible diagnosis [24–26]. Moreover, prophylactic cotrimoxazole therapy and infection with multiple opportunistic pulmonary pathogens in people with HIV/AIDS has made the diagnosis of PCP even more challenging [27]. The correlation of PCP to CD4 counts is quiet characteristic and has a bearing on making a decision regarding initiation of prophylactic therapy in HIV reactive individuals. Nearly 90-95% cases of PCP are reported in individuals with CD4 counts below 200 cells/μl, and a CD4 count bellow this threshold has in fact been cited as the strongest risk factor for PCP [6,28]. We report similar findings with majority of P. carinii associated pneumonia in our study manifesting in HIV reactive individuals with CD4 counts below 200 cells/μl. Underlying TB was noted in 10 of our patients with PCP infection. Likewise Udwadia et al., observed co-existence of TB and PCP in four (10.5%) of their study subjects [26]. Mycobacterium tuberculosis (M. tuberculosis) has in fact been reported as the most common co-pathogen seen in PCP cases [29].
Seven cases of pulmonary aspergillosis, including five cases of invasive disease were reported in this study cohort of HIV positive patients. In another research work undertaken to analyse the spectrum of AIDS related opportunistic mycoses, the authors of the present study identified four of 60 patients as probable cases of invasive aspergillosis, while one patient was diagnosed as a proven case [30]. On the other hand, Khan et al., diagnosed pulmonary aspergillosis in only four of 160 confirmed HIV positive patients with a lower respiratory tract infection [10]. A co-existing lung disease in the form of TB and/or PCP was diagnosed in five of the seven patients with pulmonary aspergillosis, highlighting the role that M. tuberculosis and P. carinii plays in damaging the lung architecture and leaving behind a scarred pulmonary parenchyma vulnerable to fungal colonization [31].
We report candida pneumonia in 8.5% of our patients, with C. albicans being the predominant Candida species isolated. Shailaja et al., obtained six isolates of C. albicans from sputa collected from 100 HIV reactive patients clinically diagnosed to have lower respiratory tract infection [32]. In a study on pulmonary bacterial and fungal infections in HIV positive patients from Mangalore, Shreevidya et al., isolated one strain of C. albicans and C. tropicalis each from sputa of HIV reactive patients [33] Similarly, Khan et al., documented candida pneumonia in four patients, with C. albicans and C. glabrata isolated from three and one patient respectively [10]. It is difficult to ascertain the clinical significance of Candida isolates in respiratory infections because of its common presence as a normal flora. A good clinical correlation is therefore essential. In our study, presence of respiratory illness in patients with repeated isolation of the same Candida species from sputa collected on more than one occasion, presence of plenty of polymorphonuclear leucocytes along with direct demonstration of budding yeast cells and pseudohyphae in sputum samples; and invasive nature of infection as evidenced by detection of Candida antigen in sera has enabled us to attribute these respiratory infections to Candidaspecies.
Lung is the portal of entry for the fungus Cryptococcus. However, the most frequent presentation of cryptococcosis in HIV positive patients is meningitis and pulmonary cryptococcal infections are generally missed due to their early and non specific symptomatology [34]. Pulmonary cryptococcal disease can manifest either alone or in conjunction with meningitis [6]. In the present study, 2.8% of the study population was diagnosed with a cryptococcal pneumonia. Khan et al., detected cryptococcal pneumonia in three of 160 HIV reactive patients [10], while in another study conducted by the present authors to describe the clinicomycological profile of HIV positive patients with suspected fungal infections, pulmonary cryptococcal disease was identified in 3.3% cases [30]. We hypothesize that cryptococcal pneumonia has all this while been an underdiagnosed entity mainly due to its non specific clinical manifestations and variable radiographic features, and suggest that this fungal agent be considered a possible differential in the aetiological profile of pneumonia in an HIV positive patient.
Thus, we observed a definite fungal aetiology in 25 (35.2%) of our patients with AIDS related opportunistic pneumonias with P. carinii being the leading causative fungal agent followed by Aspergillus species, Candida species and Cryptococcus neoformans. In contrast, Khan et al., diagnosed fungal respiratory tract infection in 14 (8.7%) of total 160 HIV reactive patients with lower respiratory tract involvement [10]. A major reason for the difference in mycological yield obtained in the two studies is the higher detection rate of P. carinii in our study versus that of Khan et al., (21.1% versus 1.8%). A factor that is likely to affect the percentage of PCP patients is the difference in the composition of the patient population in the two studies. The authors of the above mentioned study excluded all patients with confirmed diagnosis of pulmonary TB from their study population. As a result a major bulk of PCP in patients with active TB would have been missed. It is because of the above cited reason that the aetiological profile of fungal lower respiratory tract infections as observed by Khan et al., was also quite different from ours, with candida pneumonia (n=4) and pulmonary aspergillosis (n=4) being the most common, followed by PCP (n=3) and cryptococcal pneumonia (n=3). Another study conducted in Nigeria has reported Candida species to be the most common fungi recovered from sputa of adult HIV positive patients with chronic cough followed by Cryptococcus neoformans and Aspergillus species [9].
As far as Histoplasma is concerned, the infection till now has been mainly seen in endemic areas. However, case reports of histoplasmosis from India have become increasingly evidenced over the last couple of years [35,36]. It was interesting to find seroconversion and detection of histoplasma antibodies in 21(29.6%) of 71 HIV reactive patients with a clinical picture suggestive of pneumonia. It was difficult to make a definitive diagnosis of histoplasma pneumonia in these cases since antemortem deep tissue samples from suspected patients could not be obtained. We relied on antibody detection for a presumptive diagnosis of histoplasma infection, which can only act as a supportive evidence and an index of seroepidemiology of this infection. The test can be positive even in individuals with past infections and false positive reactions due to cross reactivity with other fungi such as Aspergillus, Blastomyces, Coccidioides, Candida and Cryptococcus further reduce the specificity of the test [37]. Nevertheless, documentation of seroconversion, in light of no other established aetiology in a patient with deep organ involvement such as those with lung infections; and in an immunocompromised setting of HIV positivity and low CD4 counts should raise a suspicion of histoplasmosis and these suspected infections in HIV/AIDS cases should be looked into and worked upon.
Despite best of our literary searches, we could find very few studies that have attempted to determine the frequency and the entire aetiological spectrum of fungal pneumonias in HIV/AIDS patients. Furthermore, such reports are still fewer from resource constraint settings of developing countries such as India that harbor a major burden of HIV/AIDS patients, but, where laboratory services providing mycological diagnostic facilities are limited, and much attention has been given to investigating tubercular and bacterial aetiologies of lower respiratory tract infections. Ours is one of the few prospective studies undertaken in this regard and at one of the largest tertiary care referral centres in North India. The present study has provided not only the most contemporary but also a regional profile of HIV/AIDS-related opportunistic pneumonias from this part of the country.
Limitation
The present study however, is fraught with a few limitations. Firstly, the sample size of the study group was small as a result of which we could not establish any correlation between CD4 counts of patients and occurrence of various fungal opportunistic pneumonias. Secondly, keeping in view the ethical concerns and the serious underlying condition of the patients, confirmation of results by deep tissue sampling and histopathological diagnosis could not be accomplished in many cases. We suggest that, larger, prospective studies be conducted to provide a better insight into the role that fungi play in causation of AIDS related opportunistic pneumonias so that guidelines can be formulated and appropriate CD4 cut offs can be defined for initiation of prophylactic regimens against various fungal pathogens.
Conclusion
Our study highlights that fungi contribute significantly to the aetiology of opportunistic pneumonias in HIV/AIDS patients, and that a mycological diagnosis should be strongly considered in these cases. Pneumocystis carinii was noted to be the leading fungal pathogen infecting the lungs of HIV/AIDS patients in our setting followed by Aspergillus species, Candida species and Cryptococcus neoformans. Based on our findings, we strongly emphasize on the need to include prophylactic antifungal agents as a component of management protocol of HIV reactive patients in an Indian scenario.
Source of support/funding: ICMR (Indian Council of Medical Research).
*Human immunodeficiency virus.
*Human immunodeficiency virus; † Tuberculosis; ‡ No abnormality detected.