Facial Dysmorphism: An Unreported Teratogenicity with Levetiracetam
Jyotsana Gupta1, Sandhya Jain2, Shalini Rajaram3, Neerja Goel4, Bindiya Gupta5
1 Senior Resident, Department of Obstetrics and Gynaecology, UCMS and GTB Hospital, Delhi, India.
2 Associate Professor, Department of Obstetrics and Gynaecology, UCMS and GTB Hospital, Delhi, India.
3 Director and Professor, Department of Obsetrics and Gynaecology, UCMS and GTB Hospital, Delhi, India.
4 Director and Professor, Department of Obstetrics and Gynaecology, UCMS and GTB Hospital, Delhi, India.
5 Assistant Professor, Department of Obstetrics and Gynaecology, UCMS and GTB Hospital, Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sandhya Jain, 125, SFS Apartments, Phase 4, Ashok Vihar, Delhi-110052, India.
E-mail: drsandy2015@gmail.com
Levetiracetam (LEV) is a relatively newer anticonvulsant drug used to treat epilepsy and is approved by United States Food and Drugs Administration (USFDA). The drug binds to a synaptic vesicle glycoprotein and inhibits presynaptic calcium channels, thus reducing neurotransmitter release. Commonly reported side effects include drowsiness, weakness, unsteady gait, mood changes and loss of appetite. Like most other antiepileptics, it is a Category C drug in pregnancy. We report the first case of facial dysmorphism in the neonate of a mother taking LEV antenatally. A 30-year-old lady, G2P1L1 presented at 38 weeks gestation with history of previous caesarean and leaking per vaginum. She was a known epileptic, taking carbamazepine since three to four years. She was switched over to LEV at fifth week of pregnancy. Her antenatal period was uneventful. Basic investigations including anomaly scan were normal. Unfavourable cervix necessitated caesarean section. Neonate (female) had dysmorphic facies with bilateral preauricular appendages and lateral cleft. Infantogram was suggestive of bifid vertebra in thoracic region. Computed Tomography (CT) scan chest revealed bifid vertebral body at D5 level with fusion of spinous process of D5 and D6 vertebra on left side.
This is the first case of LEV induced facial dysmorphism, highlighting the need of further studies on LEV safety during pregnancy.
Antiepileptic drugs, Bifid vertebra, Category C drug, Facial dysmorphism
Case Report
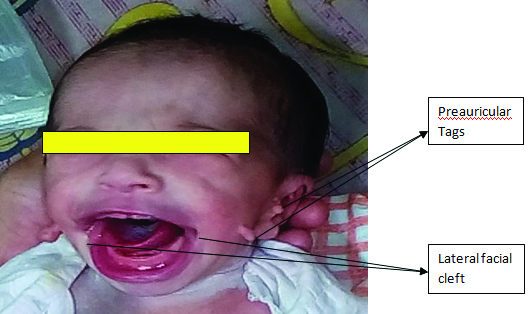
A 30-year-old female G2P1L1 presented at 38 weeks gestation with history of previous caesarean and leaking per vaginum. She was a booked case and known epileptic since childhood. Last episode of seizure was four years back and she was taking tablet carbamazepine since three to four years. She was switched over to LEV in the dose of 1000 mg/day at five weeks of gestation when she was diagnosed to be pregnant. There was no history of periconceptional folic acid intake. Her antenatal period was uneventful. Basic investigations including anomaly scan were normal. The dose of drug was increased to 1250 mg/day at 32 weeks of pregnancy; the drug levels were not monitored during pregnancy. Unfavourable cervix necessitated caesarean section. Neonate (female) had dysmorphic facies with bilateral pre-auricular appendages and bilateral lateral cleft [Table/Fig-1]. The neonate was investigated for other anomalies; blood investigations were normal. Infantogram was suggestive of bifid vertebra in thoracic region. CT scan chest revealed bifid vertebral body at D5 level with fusion of spinous process of D5 and D6 vertebra on left side. CT scan of temporal bone and ultrasound whole abdomen were normal. A diagnosis of LEV induced teratogenicity was made as: a) LEV was started during period of peak organogenesis; b) Therapeutic blood levels of carbamazepine were still there leading to polytherapy; c) Facial clefts are known to occur when insult occurs between 7-10 weeks of gestation; d) LEV levels done on day-2 postpartum were high i.e., 10 mg/l (Normal level-0.7 to 3.4 mg/l). At two months follow up, baby had difficulty in breast feeding due to poor suction because of lateral cleft, otherwise thriving well.
Levetiracetam induced facial dysmorphism.
Discussion
Prevalence of epilepsy in pregnancy is 0.3–0.6%. Incidence of major congenital malformations is 2.8% in women with untreated epilepsy which is almost similar to general population. However, there is two-three fold increased risk of congenital malformation in pregnant women taking Anti Epileptic Drugs (AEDs), especially with polytherapy [1]. The most common malformations observed secondary to in-utero AED exposure are cardiac followed by hypospadias and facial cleft [2]. The timing of exposure is crucial. Cleft lip occurs with exposure of AED ~5 weeks post conception; Cleft palate occurs with exposure 8-12 weeks post conception [3]. Older AED include phenytoin, phenobarbitone valproate and carbamazepine; Of these, sodium valproate has the maximum dose-dependent teratogenicity (11%). Newer AEDs e.g., lamotrigine, topiramate, oxcarbazepine, levetiracetam and gabapentine are relatively safe. Topiramate is found to have a higher teratogenicity amongst them (4.6%) [4]. The exact mechanism of teratogenicity is not clear; suggested mechanisms are genetic susceptibility, reactive intermediates i.e., oxidative free radicals, interference with folate metabolism, fetal hypoxia due to maternal seizures and ischemia due to cardiorespiratory depression. All these can lead to alteration in fusion of embryonic folds [5]. There is no absolutely safe dose of AED that can provide therapeutic efficacy without the potential risk of inducing developmental or structural defects in the exposed infant.
LEV is a relatively newer anticonvulsant drug, USFDA approved in 2008 used to treat epilepsy and used as monotherapy for epilepsy in the case of partial seizures, or as an adjunctive therapy for partial, myoclonic and tonic-clonic seizures. The drug binds to a synaptic vesicle glycoprotein and inhibits presynaptic calcium channels, thus reducing neurotransmitter release [6,7]. It has a linear pharmacokinetics with rapid onset of action, is totally excreted by the kidneys and does not interact with other drugs. Almost 50% fall in plasma drug levels occur in third trimester. The use of LEV as a broad-spectrum anti epileptic drug has been increasing in pregnancy owing to its better tolerability, good efficacy and predictable blood level alterations [2]. Commonly reported side effects include drowsiness, weakness, unsteady gait, mood changes and loss of appetite. However, its most serious adverse effects are behavioural [8]. Like most other antiepileptics, it is a Category C drug in pregnancy [9]. Animal studies have shown increased incidence of minor fetal skeletal abnormalities and growth retardation. No major congenital malformations have been reported in humans. Minor skeletal defects have been reported in 0-2.4% pregnant women in various clinical registries, especially with polytherapy. None of the studies have reported facial defects [10].
In North America, 5667 antenatal women on AED were studied from 1997 to 2011. Prevalence of teratogenicity with LEV was found to be 2.4% as compared to topiramate (4%), lamotrigine (2%) and oxcarbazepine (2.2%). Minor skeletal malformations were reported with LEV. In a study from Denmark conducted on 1532 women, there were no congenital malformations with LEV as compared to topiramate 4.6%, lamotrigine 3.7% and oxcarbazepine 2.8%. A study in the Journal Neurology retrospectively looked at 671 human pregnancies with known maternal exposure to LEV and found that the rate of major congenital malformations were not significantly higher when LEV was used as a monotherapy. However, the majority of the patients were also exposed to other AEDs as a combination therapy and found increase in major congenital malformations when combined with valproate and carbamazepine. The paper concluded that the data suggests LEV monotherapy to be a suitable regimen if anti-epileptic medication is needed during pregnancy [11]. A study by Mawhinney E et al., reported that mean daily dose of LEV that leads to major malformations was 3,000 mg as against 1,680 mg for minor malformations and 1,148 mg for no malformations [10].
During pregnancy there occurs alteration in pharmacokinetics of AEDs due to physiological increase in plasma volume, impaired drug absorption e.g., in hyperemesis gravidarum, enhanced metabolic elimination through enzyme induction, decreased drug binding to plasma proteins and enhanced renal elimination. In the management of epilepsy the general principles are reconsider the need for continued AED medication if patient is seizure free for few years; any alterations in the drug type and dose should be done prior to conception; consider monotherapy at lowest effective dose; periconceptional folic acid; valproate is to be avoided; determine optimal prepregnancy AED blood level as standard/baseline for monitoring during pregnancy; clinical monitoring and blood level monitoring of AED in each trimester and post partum readjustment of the dose. Benefits of breast feeding outweigh the potential risk with AED in neonate and they are mostly safe. Exposure depends on maternal plasma concentration, fraction of AED transferred, neonatal absorption and elimination capacity. AED with low protein binding, low molecular weight and high lipophilicity are likely to accumulate in breast milk. Carbamazepine, phenobarbitone, oxcarbazepine, lamotriagine and topiramate have low to moderate protein binding. LEV has no protein binding and is 100% transferred to neonate but blood levels are low due to effective elimination [12].
Conclusion
This case is being reported as a first case of LEV induced facial dysmorphism. This case warrants the need of further studies for use of LEV as antiepileptic drug in pregnancy.
[1]. Artama M, Auvinen A, Raudaskoski T, Antiepileptic drug use of women with epilepsy and congenital malformations in offspringNeurology 2005 64:1874-78. [Google Scholar]
[2]. Hill DS, Wlodarczyk BJ, Palacios AM, Finnell RH, Teratogenic effects of antiepileptic drugsExpert Rev Neurother 2010 10(6):943-59. [Google Scholar]
[3]. Nulman I, Laslo D, Koren G, Treatment of Epilepsy in PregnancyDrugs 1999 57(4):535-44. [Google Scholar]
[4]. Hernández-Díaz S, Smith CR, Shen A, Mittendorf R, Hauser WA, Yerby M, Comparative safety of antiepileptic drugs during pregnancyNeurology 2012 78:1692 [Google Scholar]
[5]. Etemad L, Moshiri M, Moallem SA, Epilepsy drugs and effects on fetal development: Potential mechanismsJ Res Med Sci 2012 17(9):876-81. [Google Scholar]
[6]. Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetamProc Natl Acad Sci USA 2004 101(26):9861-66. [Google Scholar]
[7]. Vogl C, Mochida S, Benjamin WC, Whalley J, Stephens GJ, The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathwayMol Pharmacol 2012 82(2):199-208. [Google Scholar]
[8]. Clinical Epilepsy: Pediatrics”Epilepsia 2005 46(8):142-67. [Google Scholar]
[9]. “Highlights of prescribing information”(pdf). Ucb, inc. Retrieved 29 may 2014 [Google Scholar]
[10]. Mawhinney E, Craig J, Morrow J, Russell A, Smithson WH, Parsons L, Levetiracetam in pregnancy: Results from the UK and Ireland epilepsy and pregnancy registersNeurology 2013 80(4):400-05. [Google Scholar]
[11]. http://www.drugs.com/news/study-weighs-safety-epilepsy-pregnancy-49908.html [Google Scholar]
[12]. Sabers A, Tomson T, Managing antiepileptic drugs during pregnancy and lactationCurr Opin Neurol 2009 22:157-61. [Google Scholar]