Urinary Uric Acid/Creatinine Ratio - A Marker For Perinatal Asphyxia
Kinjal Prahaladbhai Patel1, Mayur Goradhanbhai Makadia2, Vishwal Indravardan Patel3, Haridas Neelakandan Nilayangode4, Somashekhar Marutirao Nimbalkar5
1 Resident, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India.
2 Resident, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India.
3 Resident, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India.
4 Professor, Department of Biochemistry, Pramukhswami Medical College, Karamsad, Gujarat, India.
5 Professor, Department of Paediatrics, Pramukhswami Medical College, Karamsad, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Somashekhar Marutirao Nimbalkar, Professor, Department of Paediatrics, Pramukhswami Medical College, Karamsad-388325, Gujarat, India.
E-mail: somu_somu@yahoo.com
Background
Perinatal hypoxia is one of the leading causes of perinatal mortality in developing countries. Both apgar score and arterial blood pH predict the neonatal mortality in asphyxia. Apgar score alone does not predict neurologic outcome and as it is influenced by various factors. This study was conducted to evaluate the utility and sensitivity of urinary uric acid to creatinine ratio (UA/Cr ratio) in asphyxia diagnosis, compared to invasive Arterial Blood Gas (ABG) analysis.
Aim
To assess the urinary uric acid/creatinine ratio as an additional marker for perinatal asphyxia compared with ABG analysis in apgar score monitoring.
Materials and Methods
The present case control study was conducted at a teaching hospital in Central Gujarat. Data of 40 healthy newborns and 40 asphyxiated newborns were collected. In absence of regional estimates, a sample of size 39 was required to attain a power of 80% at 5% alpha (type I error) considering a moderate effect size of 0.65. (UA/Cr) ratio was measured from the spot urine sample collected during 24-72 hours of birth. Statistical analysis was performed by Independent t-test, Pearson’s correlation coefficient (r) and Receiver Operating Characteristic (ROC) plots.
Results
The mean (UA/Cr ratio) (2.75±0.18 vs 1.78±0.23) is significantly higher in asphyxiated group than in the control group (p<0.0001). Urinary UA/Cr ratio had negative correlation with blood pH (r= -0.27, p=0.18), which was not significant (p>0.05). Urinary UA/Cr ratio with criterion of >2.3 had 100% sensitivity, 100% specificity with AUC of 1 (p<0.0001) had a better predictive value.
Conclusions
Apgar score is usually reduced in neonates with congenital anomalies and premature neonates. Hence, it is preferable that the clinical diagnosis of asphyxia by apgar scores be supported by other investigations so that early decision can be taken about the level of care the baby needs. pH, lactates and base deficits change with establishment of respiration following resuscitation. However, pH, lactate, base deficit estimations are invasive and need rapid estimations. Non-invasive urinary UA/Cr ratio may be an answer to these issues as it easy, economical and equally efficient.
Asphyxia markers, Hypoxic-ischemic encephalopathy, Neonatal mortality
Introduction
Cerebral blood flow and gas exchange is compromised in perinatal asphyxia leading to acidosis, hypercapnea and hypoxia. The poor outcomes thus result in high neonatal mortality rates as well as high morbidity rates in developing countries [1,2]. The diagnosis and grading of asphyxia can be difficult especially if relevant information at the time of delivery is not available [3,4]. There is ample literature available that has improved our understanding of the mechanisms that lead to birth asphyxia [5], but early indicators of tissue damages due to birth asphyxia are lacking and not widely studied. Brief hypoxia damages cerebral oxidative metabolism leading to an anaerobic glycolysis, yielding only 2 molecules of Adenosine Triphosphate as compared to 32 molecules of ATP during aerobic conditions [6]. Prolonged hypoxia, produces further failure of oxidative phosphorylation and ATP production. Lack of ATP and increased cellular destruction will cause an accumulation of Adenosine Monophosphate (AMP) and Adenosine Diphosphate (ADP), which will then get catabolised to its constituents of adenosine, inosine and hypoxanthine [7,8]. Continuous tissue hypoxia and consequent reperfusion injury will result in hypoxanthine being oxidized to xanthine and uric acid in presence of xanthine oxidase. This will increase the uric acid production and cause it to enter blood from damaged tissues. This uric acid will then get excreted in urine where it can be easily detected. There are also over one million neonatal deaths per year in India which is 25% of the total global burden of neonatal deaths. The overall neonatal mortality rate in India is 28/1000 live births [9]. The most commonly used diagnostic and prognostic index to evaluate asphyxia in neonate is apgar score [10]. But apgar score alone is not useful to ferret out neurologic outcome, which is influenced by various factors like immaturity, foetal malformations, maternal medications and infection [11].
Accurate appraisal of late neurological consequences has failed by performing policies such as foetal heart monitoring [12], Apgar score [13]. While analysis of xanthine, hypoxanthine, and some inflammatory cytokines are time consuming, costly and not routinely available for clinical care [14–17].
We set out to assess the urinary ratio (UA/Cr) in relation to apgar score and arterial blood gas analysis, as a marker for perinatal asphyxia in our clinical setting.
Materials and Methods
The study was carried out in the Department of Paediatrics of a University Medical College in Gujarat. The neonatal intensive care unit (NICU) is Level 3 and also runs a fellowship program in neonatology and has ability to do cooling for perinatal asphyxia as well as advanced capabilities of ventilation such as nitric oxide and high frequency ventilation. All deliveries are attended by residents trained in neonatal resuscitation. Eighty neonates were recruited for the study. Forty term babies with 35 weeks of gestations with apgar score >/=7 at 5 min with no signs of asphyxia were recruited as controls and 40 term babies with >35 weeks of gestations admitted to NICU with apgar score of 6 or less at 5 minutes of birth were recruited as cases. In absence of regional estimates, a sample of size 39 was required to attain a power of 80% at 5% alpha (type I error) considering a moderate effect size of 0.65. Babies with congenital malformations, suspected metabolic disease on treatment with diuretics, suffering from anuria and those born to mothers having hypertension, diabetes mellitus, toxaemia of pregnancy, receiving general anaesthesia, pethidine, phenobarbitone and other drugs likely to cause depression in babies. The neonates that were included in the study did not undergo therapeutic hypothermia before the collection of the urinary sample. Mother with febrile attack within 2 months before delivery was excluded from study. The spot urinary samples within 72 hours of birth were collected by staff nurse in sterilized disposable urine bag and analyzed in hospital laboratory for UA/Cr ratio. Urinary uric acid was estimated by auto analyzer by spectrophotometric uricase method. Urinary creatinine was estimated in same above instrument by using Jaffe’s alkaline picrate method. ABG values from ABG GEM Premier were taken.
Analysis was performed using the commercially available statistical Software - STATA (14.2), and Excel 2016. The p-value of less than 0.05 was considered statistically significant. Pearson correlation coefficient was used to test the strength of association between arterial blood pH and other variables. Receiver Operating Characteristic (ROC) plots were used to determine the cut-off values of various parameters.
Ethical clearance was obtained by institution’s Human Research Ethics Committee.
Results
There were 21 females and 19 males in both groups. The mean gestational age and birth weight of asphyxiated group (35.79±1.78 weeks, 2.31±0.65 kg) were significantly lower than the control group (38.25±0.98 weeks, 2.80±1.96 kg) respectively (p<0.05). Details of baseline characteristics are given in [Table/Fig-1].
Baseline characteristics.
| Parameter | CONTROLS (N=40)Mean* (SD) | CASES (N=40)Mean*(SD) |
|---|
| Birth weight | 2.80* (1.96) kg | 2.31* (0.65) kg |
| Gestational age | 38.25* (0.98) weeks | 35.79* (1.78) weeks |
| Apgar at 5th minute | 8.25* (0.74) | 5.33* (0.66) |
| Gender (Frequency %) | 21(F)=52.5% | 21(F)=52.5% |
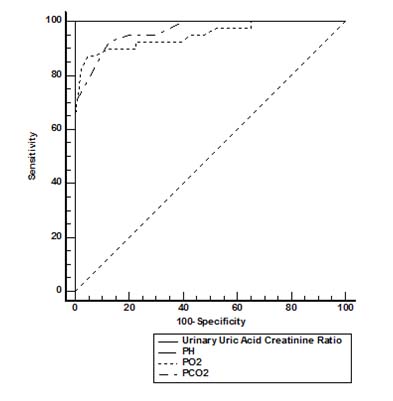
Urinary UA/Cr ratio showed negative correlation with arterial blood pH (r= -0.18, p=0.2713), which was not significant. Mean of various parameters like Apgar 5th min, arterial blood pH, Partial Pressure of Oxygen (pO2) (mm Hg) values (5.33, 7.04, 37.13) respectively were significantly lower in asphyxiated group than control group-values (8.25, 7.39, 58.70) respectively (p<0.0001). Mean of various parameters like Partial Pressure of Carbondioxide (PCO2) (mm Hg), urinary uric acid (mg/dl), and urinary uric acid/ creatinine ratio values (48.43, 37.72, 2.75) respectively were significantly higher in asphyxiated group than control group-values (40.78, 20.19, 1.78) respectively (p<0.0001). Apgar 5th min, arterial blood pH, pO2, pCO2, urinary uric acid, urinary creatinine, UA/Cr ratio between asphyxiated newborns, and healthy newborns are seen [Table/Fig-2]. The ROC curve analysis of the parameter studied is given in [Table/Fig-3] and [Table/Fig-4].
Comparison of various parameters between the control and asphyxiated group.
| Parameter | Axphyxiated Group (N=40)Mean*(SD) | Control Group (N=40)Mean*(SD) | p-value | 95% confidence intervalof the differences |
|---|
| Apgar 5th min | 5.33* (0.66) | 8.25* (0.74) | p<0.0001 | -3.2368 to -2.6132 |
| Arterial blood pH | 7.04* (0.06) | 7.39* (0.03) | p<0.0001 | -0.3672 to -0.3248 |
| pO2 (mm Hg) | 37.13* (8.80) | 58.70* (10.35) | p<0.0001 | -25.8523 to -17.2977 |
| pCO2 (mm Hg) | 48.43* (4.02) | 40.78* (2.82) | p<0.0001 | -9.1960 to -6.1040 |
| Urinary uric acid (mg/dl) | 37.72* (5.17) | 20.19* (1.24) | p<0.0001 | -19.2033 to -15.8557 |
| Urinary creatinine (mg/dl) | 13.74 (2.03) | 13.29 (3.62) | p=0.4914 | -0.8528 to 1.7603 |
| Urinary uric acid/ creatinine ratio | 2.75* (0.18) | 1.78* (0.23) | p<0.0001 | -1.0620 to -0.8795 |
ROC curve analysis-The predictive values of biochemical parameters for Perinatal asphyxia- Arterial blood pH, pO2, pCO2, and UA/Cr ratio.
| Parameter | Cut off value | Sensitivity | Specificity | AUC | P-value | 95% confidence interval of AUC |
|---|
| pH | 7.2 | 100 | 100 | 1.000 | <0.0001 | 0.955, 1.000 |
| pO2 | 42 | 87.5 | 95 | 0.949 | <0.0001 | 0.875, 0.986 |
| pCO2 | 44 | 82.6 | 87.6 | 0.964 | <0.0001 | 0.897, 0.993 |
| Urinary Uric Acid/Creatinine ratio | 2.3 | 100 | 100 | 1.000 | <0.0001 | 0.955, 1.000 |
| Apgar at 5th min. | 6 | 100 | 100 | 1.000 | <0.0001 | 0.955, 1.000 |
Discussion
Low apgar score is commonly used to as a indicator of asphyxia in infants, but it may often be not available and may be reduced in premature infants [11]. Other investigations that support the diagnosis of asphyxia would be required to improve availability of therapy. pH values are quickly normalized after the onset of respiration, due to the elimination of carbon dioxide and cannot be relied upon in patients that are transported. Additionally, lactate and base deficit are closely interconnected. The current study asserts that, urinary UA/Cr ratio >2.3 in spot urine sample within 72 hours of life had 100% sensitivity, 100% specificity with AUC of 1.000 (p<0.0001) is a better marker for perinatal asphyxia. Increased urinary UA/Cr ratio can thus be considered a useful investigation for impaired oxygen delivery in the newborn as it reflects an increased ATP degradation [18].
Urinary UA/Cr ratio is simple, non-invasive, painless and economical investigation for the diagnosis of perinatal asphyxia. Combined use of arterial blood pH, apgar scores and UA/Cr ratio can help in early decision making about the level of care the new born requires. There have been very few studies from developing countries that have focused on this parameter. Basu et al., showed that the urinary UA/Cr ratios were significantly higher in cases than controls (3.1±1.3 vs 0.96±0.54; p < 0.001) and among asphyxia patients [19], a significant negative linear correlation was found between the UA/Cr ratio and the apgar score (r = -0.857, p < 0.001). Current study is in consonance with many other studies that have looked at low apgar score and urinary UA/Cr ratio [4,7,12,18,20].
Bader D et al., found a significant correlation between urinary UA/Cr ratio and apgar score (r = 0.86, p < 0.01). This result is not in agreement with our result. On the other hand, they reported that the UA/Cr was higher in the asphyxiated group when compared to controls. (2.06 + 1.12, vs. 0.64 + 0.48; p < 0.001), these are similar type findings with our results [21]. A comparison of the various similar studies which shown in [Table/Fig-5] [18,20–22].
Studies that have comparable effects to current study [18,20–22].
| Study | Author | Results And Primary Outcomes | Reciprocity With Our Findings |
|---|
| The UA/Cr Ratio is An Adjuvant Marker for Perinatal Asphyxia | Bhongir, et al., [18] | In Asphyxiated and control group, mean urinary UA/Cr ratio was (2.58±0.48 vs 1.89±0.59). This is significant.The umbilical cord blood pH had significant positive correlation with 1st minute Apgar score (r= 0.41, p=0.003), 5th minute Apgar (r= 0.44, p=0.002), while urinary UA/Cr ratio had significant negative correlation with cord blood pH (r= -0.63, p=0.002). Urinary UA/Cr ratio with criterion of >2.43 had 80% sensitivity, 87.5% specificity with AUC of 0.84 (p=0.003) had a better predictive value. | In current study we found a negative correlation between arterial blood pH and Urinary UA/Cr ratio, which is not significant (r= -0.18, p=0.2713). |
| Urinary UA/Cr ratio as an additional marker of perinatal asphyxia. | Chen HJ et al., [20] | Urinary UA/Cr ratio was significantly higher in asphyxiated term and premature infants with and without perinatal asphyxia than term healthy infants (p<0.05). When the urinary ratio of UA to Cr was greater than 0.95, perinatal asphyxia was identified with a sensitivity of 80% and a specificity of 71% in term infants. In premature infants, a cut-off value of UA/Cr for perinatal asphyxia of 2.9 had a sensitivity of 71% and a specificity of 70%. | This is similar type study with our study. They also include premature infants. |
| Neonatal urinary uric acid/creatinine [correction of creatinine] ratio as an additional marker of perinatal asphyxia. | Bader D. et al., [21] | The UA/Cr was higher in the asphyxiated group when compared to controls. (2.06 + 1.12, vs. 0.64 + 0.48; p<0.001). Within the perinatal asphyxia group, a significant correlation was found between the UA/Cr ratio and the asphyxia score. (r = 0.86, p<0.01). | These results are well similar to our results. |
| Importance of blood gas measurement in perinatal asphyxia and alternatives to restore the acid base balance status to improve the new born performance. | H. Orozco-Gregorio et al., [22] | Piglets with lesser viability at birth had increased blood pCO2 and blood lactic acid concentrations and decreased blood pH. Moreover, the ability to thermoregulate during an acute cold stress was inversely related to umbilical blood lactate concentrations. | This study done on Piglets. But, all the conclusions and hypothesis are in sync with our study. |
Limitation
Limitations of our study are methodological in nature. More details on asphyxiated neonates such as duration of labor, duration of resuscitation, time to first breath, etc may throw more light on the utility and limitations of the lab tests. We are also limited by the fact that this is single center study with a relatively small sample size. Multicentric studies with more sample size will allow us to utilize more variables in multivariate analysis and further improve upon the predictive value of this marker.
Conclusion
Urinary UA/Cr ratio is an accessible, non-invasive, painless and cost-effective additional framework with good predictive value for diagnosing perinatal asphyxia. There exists still a need to study these parameters in the context of therapeutic hypothermia and how the parameters change over the period of treatment.
[1]. Dutta DC, Konar H, Diseases of the Fetus and the Newborn. In: Dutta DC, Konar H, editorsText book of obstetrics including perinatology and contraception 1998 4 edCalcuttaNew Central Book Publisher:504-05. [Google Scholar]
[2]. Sykes GS, Molloy PM, Johnson P, Gu W, Ashworth F, Stirrat GM, Do Apgar scores indicate asphyxia?Lancet 1982 1(8270):494-96. [Google Scholar]
[3]. Portman RJ, Carter BS, Gaylord MS, Murphy MG, Thieme RE, Merenstein GB, Predicting neonatal morbidity after perinatal asphyxia: A scoring systemAm J ObstetGynecol 1990 162:174-82. [Google Scholar]
[4]. Harkness RA, Simmonds RJ, Coade SB, Lawrence CR, Ratio of the concentration of hypoxanthine to creatinine in urine from newborn infants: A possible indicator for the metabolic damage due to hypoxiaBr J Obstet Gynaecol 1983 90(5):447-52. [Google Scholar]
[5]. Snyder EY, Cloherty JP, Perinatal Asphyxia. In: Cloherty JP, Stark Ann R, editorsManual of Neonatal Care 1998 4edPhiladelphiaLippincott-Raven Publishers:530 [Google Scholar]
[6]. Rich PR, The molecular machinery of Keilin’s respiratory chainBiochemical Society Transactions 2003 31(Pt 6):1095-105. [Google Scholar]
[7]. Manzke H, Von Kreudenstein PS, Dorner K, Kruse K, Quantitative measurements of the urinary excretion of creatinine, uric acid, hypoxanthine and xanthine, uracil, cyclic AMP, and cyclic GMP in healthy newborn infantsEur J Pediatr 1980 133:157-61. [Google Scholar]
[8]. Deorari A, Paul Vinod K, Aggarwal R, Aggarwal R, Upadhyay A, Chawla D, National Neonatal-Perinatal DatabaseNational Neonatology Forum NNPD Netw 2003 [Google Scholar]
[9]. Bank W. Mortality rate, neonatal (per 1,000 live births) [Internet]. Data. World Bank; [cited 2016Aug30]. Available from: http://data.worldbank.org/indicator/sh.dyn.nmrt [Google Scholar]
[10]. Casey BM, McIntire DD, Leveno KJ, The continuing value of the Apgar score for the assessment of newborn infantsN Engl J Med 2001 344(7):467-71. [Google Scholar]
[11]. American Academy of PediatricsThe APGAR scoreAdvances in Neonatal Care 2006 6(4):220-23. [Google Scholar]
[12]. Manzke H, Dörner K, Grünitz J, Urinary hypoxanthine, xanthine and uric acid excretion in newborn infants with perinatal complicationsActa Paediatr Scand 1977 66(6):713-17. [Google Scholar]
[13]. Nelson KB, Dambrosia JM, Ting TY, Grether JK, Uncertain value of electronic fetal monitoring in predicting cerebral palsyN Engl J Med 1996 334(10):613-18. [Google Scholar]
[14]. Tekgul H, Yalaz M, Kutukculer N, Ozbek S, Kose T, Akisu M, Value of biochemical markers for outcome in term infants with asphyxiaPediatr Neurol 2004 31(5):326-32. [Google Scholar]
[15]. Naithani M, Simalti AK, Biochemical Markers in Perinatal AsphyxiaJ Nepal Paediatr Soc 2011 31(2):151-56. [Google Scholar]
[16]. Lin M, Chou H, Chen C, Tsao P, Hsieh W, Early serum biochemical markers and clinical outcomes in term infants with perinatal asphyxia or low apgar scoresClin Neonatol 2008 15(1):10-5. [Google Scholar]
[17]. Law JA, Panagiotopoulos C, Derrik EJ, Newborn complications after intrapartum asphyxia with metabolic acidosis in the term fetusAm J Obstet Gynecol 1994 170:1081-87. [Google Scholar]
[18]. Bhongir AV, Yakama AV, Saha S, Radia SB, Pabbati J, The Urinary Uric Acid/Creatinine Ratio is An Adjuvant Marker for Perinatal AsphyxiaEur J Pharm Med Res 2015 2(5):520-28. [Google Scholar]
[19]. Basu P, Som S, Choudhuri N, Das H, Correlation between apgar score and urinary uric acid to creatinine ratio in perinatal asphyxiaIndian journal of clinical biochemistry 2008 23(4):361-64. [Google Scholar]
[20]. Chen HJ, Yau KI, Tsai KS, Urinary uric acid/creatinine ratio as an additional marker of perinatal asphyxiaJ Formos Med assoc 2000 99:771-74. [Google Scholar]
[21]. Bader D, Gozal D, Weinger-Abend M, Berger A, Lanir A, Neonatal urinary uric acid/creatinine [correction of creatinine] ratio as an additional marker of perinatal asphyxiaEur J Pediatr 1995 154(9):747-49. [Google Scholar]
[22]. Orozco-Gregorio D, Mota-Rojas M, Alonso-Spilsbury M, González-Lozano M, Trujillo-Ortega SA, Olmos-Hernández P, Importance of blood gas measurements in perinatal asphyxia and alternatives to restore the acid base balance status to improve the newborn performanceAm J Biotechnol Biochem 2007 3(3):131-40. [Google Scholar]