HAI defined as “the infections acquired in hospital by a patient who was admitted for a reason other than that infection, in whom the infection was not present or incubating at the time of admission and symptoms should appear at least after 48 hours of admission” [1,2]. According to the data published by World Health Organization (WHO), on “The Burden of Health-Care Associated Infection Worldwide” from 1995 to 2008, the overall prevalence of HAI in developed countries varied between 5.1% and 11.6%. The burden of HAI is much higher in developing countries and among high-risk populations, such as patients admitted in critical care units and neonates [3]. Occurrence of HAIs in hospitalised patients is affected by multiple factors like location of the hospital such as type of Intensive Care Units (ICUs) or ward, patient population, immunity status, underlying comorbidities like hypertension, diabetes, COPD, and chronic kidney disease. Nevertheless hospital infection control practices and the most importantly the hospital administration play a crucial role [4,5].
HAIs have been shown to occur about 5 to 10 times more in the patients admitted in ICUs than in the general wards [6]. Various factors may contribute to the increased incidence of HAIs in ICUs such as: i) Increased device use such as mechanical ventilators, urinary catheters and central line in the ICUs than in wards; ii) ICU patients are critically ill and more often immuno-compromised compared to those in general wards [7]. In addition as most ICU patients are frequently on broad spectrum antimicrobials, this induces selective antibiotic pressure which leads to development of AMR among the microorganisms of ICUs [8]. Hence, the microbiology profile of the HAIs in the ICUs often reveals multidrug resistant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus including MRSA, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) [9–12]. Presence of HAIs in ICU patients causes a statistically significant increase in the length of hospital stay, mortality and financial burden [13].
AMR is on the rise and is a serious threat to global public health. Hence to prevent AMR, our first and foremost goal should be to use antimicrobials judiciously. AMR surveillance helps us to generate such information by providing a baseline data on pattern of microorganisms in the hospital and their susceptibility pattern which helps to choose the appropriate antimicrobials [14].
AMR surveillance is the most important tool for assessing the burden of AMR and for providing the necessary antibiogram data, based on which the local, national and global treatment strategies can be planned. The Global Antimicrobial Resistance Surveillance System (GLASS) has been launched in May 2015 by WHO to support a standardised approach for AMR data collection, analysis and sharing at a global level [15].
Many studies on AMR surveillance are available in developed countries. But unfortunately studies on AMR surveillance are not adequate from developing countries, including India. Recently, ICMR (Indian Council of Medical Research) has created an Antimicrobial Resistance Surveillance Network, which included only five major hospitals of India. As this kind of data is scarce in current times in Southern India hospitals, this study was undertaken with the objective of studying the occurrence of different types of HAIs and the AMR pattern of the bacterial pathogens isolated from patients admitted to various ICUs of JIPMER. This baseline knowledge may provide necessary information to formulate antibiotic policy in our tertiary care hospital.
Materials and Methods
This is a record based cross-sectional study, carried out at the office of HICC (Hospital Infection Control Committee) and at the Department of Microbiology at JIPMER. Total of 14 ICU’s with an overall bed strength of nearly 300 were included in our study. ICUs are categorised into adult medical ICUs such as Medicine ICU (MICU), Medical oncology ICU, Critical Care ICU (CCU) and Neuromedicine ICU (N-MICU); adult surgical ICUs which includes Cardiothoracic and Vascular Surgery ICU (CTVS ICU), Kidney transplantation ICU (KTP ICU), Surgical Gastroenterology ICU (SGE ICU) Urology ICU, Plastic Surgery ICU, Neurosurgery ICU and Surgery ICU (SICU) and the paediatric ICUs such as, Paediatrics ICU (PICU) and Neonatal ICU (NICU) and Paediatric surgery ICU.
Retrospective data of culture reports of the patients admitted to all the ICUs of JIPMER during the period from April 2015 to March 2016 were collected.
A total of 5,595 patients were admitted to the ICU during the study period; of whom 1,244 patients were shown to have developed HAIs as their clinical specimens were found to be culture positive. The remaining ICU patients whose samples were negative by culture were excluded from the study. A total of 4,764 isolates were obtained from the clinical specimens of 1,244 patients. More than one isolate of the same organism from the same patient were excluded, following this the number of isolates was 3,090; which was used for analysis. Patients with incomplete case records were also excluded from this study.
The culture and identification was carried out on the clinical specimens received from ICU patients in the Department of Microbiology as a routine procedure according to the Standard Operating Procedures (SOP) of the department. Culture media used for isolation of these organisms were blood agar, MacConkey agar and chocolate agar. Identification was done based on the colony morphology and biochemical tests. Antimicrobial susceptibility testing was performed using the Kirby-Bauer disk-diffusion method and was reported according to Clinical Laboratory Standards Institute (CLSI) guidelines [16]. The antimicrobial agents tested were- gentamycin (10 μg), amikacin (30 μg), ceftriaxone (30 μg), ceftazidime (30μg), cefoperazone+sulbactam (75/10μg), ciprofloxacin (5 μg), meropenem (10 μg), for all gram negative bacterial isolates. For Staphylococci, penicillin (10 units), erythromycin (15 μg), clindamycin (2μg) ciprofloxacin (5μg), gentamycin (10μg), cefoxitin (30μg), co-trimoxazole (25μg) were tested. For Enterococci- high level Gentamycin (120μg), ampicillin (10μg), tetracycline (30μg) and vancomycin (30μg) were employed. Oxacillin screen agar and cefoxitin disk diffusion tests were used for screening for methicillin resistance among Staphylococci and vancomycin screen agar for screening vancomycin resistance among both Staphylococci and Enterococci as per SOP manual.
Culture sensitivity reports of the patients admitted to various ICUs for the duration of twelve months (April 2015- March 2016) were collected from Hospital Information System (HIS). Only the reports that satisfied the inclusion criteria were taken for data analysis.
Parameters such as gender and age of the patient, type of the ICU, infecting organism, site of infection, type of HAIs, and antimicrobial resistance pattern including different co-resistance were extracted for all the study subjects from the available hospital record.
Detection of MDR Gram-Negative Bacilli:
MDR organism was defined as “resistance to at least 3 different antibiotic groups, as reported elsewhere” [17]. Only those antimicrobial agents were included in the present study for analysis if the susceptibility testing had been performed for at least 80% of the isolates [17]. According to these criteria, MDR has been defined for clinically significant major GNB such as Acinetobacter species, Pseudomonas species, Escherichia coli, and Klebsiella species. Classes of antibiotics used for MDR-GNB analysis were Aminoglycosides (AMG), Cephalosporins (CEPH), Carbapenems (CARB), Tetracyclines (TETRA) and Fluroquinolones (FQ).
5 MDR (AMG+ CEPH+ FQ+ CARB+ TETRA)- Resistant to five classes of antimicrobials such as aminoglycosides, third generation cephalosporins, fluoroquinolones, carbapenems, and tetracyclines. This pattern of MDR is analysed only for Acinetobacter spp as tetracyclines are tested only for Acinetobacter spp among MDR GNB.
4 MDR (AMG+ CEPH+ FQ+ CARB)- Resistant to four classes of antimicrobials such as aminoglycosides, third generation cephalosporins, fluoroquinolones and carbapenems.
3 MDR-
3 MDR (AMG+ CEPH+ FQ)- Resistant to three classes of antimicrobials such as aminoglycosides, third generation cephalosporins and fluoroquinolones but sensitive to carbapenems.
3 MDR (CARB+ CEPH+ FQ)- Resistant to three classes of antimicrobials such as third generation cephalosporins, fluoroquinolones and carbapenems but sensitive to aminoglycosides.
3 MDR (AMG+ FQ+ CARB)- Resistant to three classes of antimicrobials such as aminoglycosides, fluoroquinolones and carbapenems, but sensitive to third generation cephalosporins.
3 MDR (AMG+ CEPH+ CARB)- Resistant to three classes of antimicrobials such as aminoglycosides, third generation cephalosporins and carbapenems, but sensitive to fluoroquinolones.
Note: For testing against third generation cephalosporins; ceftriaxone and ceftazidime were used except for Acinetobacter and Pseudomonas species, where only ceftazidime was used. For testing for carbepenems, meropenem was used; for aminoglycosides, gentamycin and amikacin were used and for fluoroquinolones, ciprofloxacin was used.
Ethics
The study protocol was approved by JIPMER Undergraduate Research Monitoring Committee (UGRMC) followed by approval taken from Institute Ethics Committee (IEC).
Statistical Analysis
Data were entered and analysed using Microsoft Excel. Continuous variables like age were expressed as mean±SD. Categorical variables like proportion of bacterial infections across different ICUs, age groups, gender were expressed as percentages. Pattern of micro-organisms and age groups/type of ICUs/sites of infections were analysed and expressed as percentages.
Results
In the present study, a total of 3,090 isolates obtained from the clinical specimens of 1,244 patients were used for data analysis.
Demographic Characteristics:
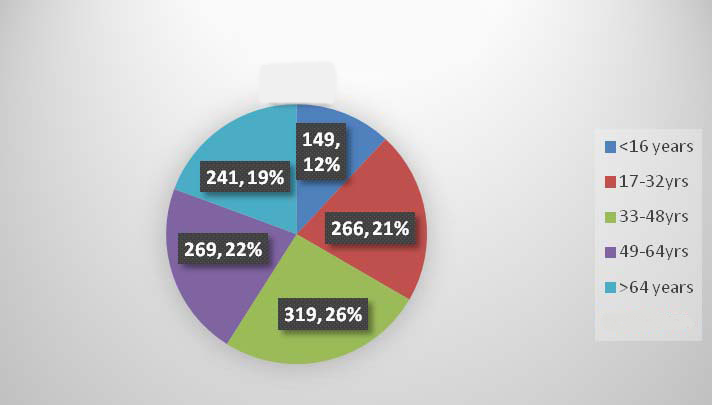
Demographic characteristics among the patients under this study are shown in [Table/Fig-1]. We noted that most of the patients were between the age group of 33-48 years; followed by 49-64 years. Males 64% (792/1244) were predominant among admitted ICU patients.
Distribution among various age groups.
Total patients - 1244
Clinical specimen and organisms isolated:
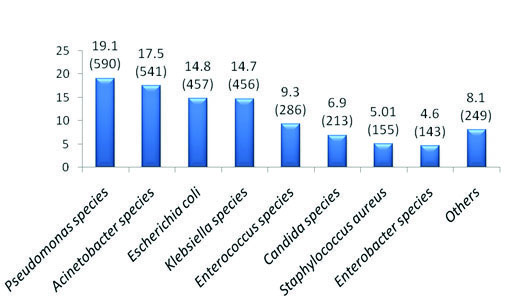
Most common culture positive clinical specimen received during the present study was tracheal aspirate (29.9%) followed by exudate (22.7%) and urine (19.5%). Distribution of various clinical specimens received from the ICUs during the study period is shown in [Table/Fig-2]. It was observed that Pseudomonas spp (19.09%) was the most common organism isolated from various clinical specimens in our study followed by Acinetobacter spp (17.5%) [Table/Fig-3]. Other common organisms isolated in decreasing order were Escherichia coli (14.8%), Klebsiella spp (14.7%), Enterococcus spp (9.3%) and Candida spp (6.9%).
Distribution of clinical specimens which are found to be culture positive (n = 3090).
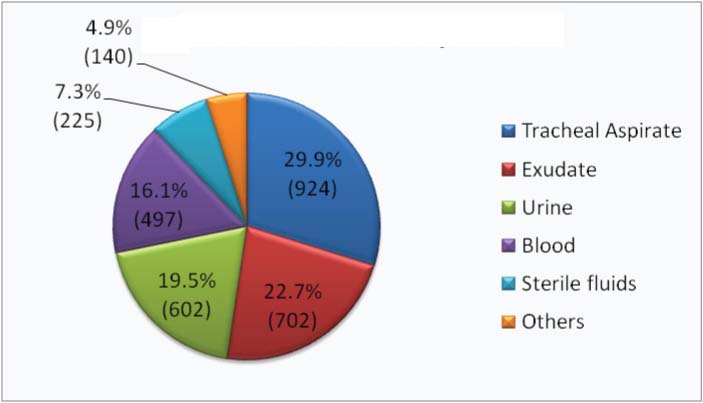
Distribution of isolates (%) among various clinical specimens (total no. of isolates 3090).
Attempt was made to find out the most common pathogen predominant in individual ICUs [Table/Fig-4]. Klebsiella spp, Acinetobacter spp, Candida spp were higher among the various Paediatric ICUs. In Adult ICUs, Pseudomonas spp, Acinetobacter spp, Escherichiacoli and Klebsiella spp were increasingly isolated.
ICU wise distribution of clinical isolates (Percentage).
| NICU | PICU | Ped SG ICU | MICU | CCU | NM ICU | Onco ICU | Uro ICU | SICU | CTVS ICU | NSG ICU | PSG ICU | SGE ICU |
|---|
| Klb 29.7 | Aci 21.2 | Cnd12.3 | Aci 21.7 | Aci 24.9 | Psd 24.1 | Psd 24.4 | Esh 37.68 | Aci 17.2 | Psd 28.7 | Aci 23.8 | Psd 18.7 | Esh 20.7 |
| Sta 13.3 | Psd 18.1 | Klb16.5 | Psd 21.4 | Psd 18.8 | Aci 19.4 | Klb 20.7 | Psd 18.84 | Psd17 | Klb 16.2 | Psd 21.8 | Esh 18.7 | Klb 16.4 |
| Enc 12.7 | Cnd 11.9 | Enc16.5 | Klb 12.6 | Esh 13 | Klb 12 | Sta 10.9 | Klb 15.94 | Esh 15.7 | Aci 15 | Klb 16.6 | Klb 17.3 | Enc 16.4 |
| Aci 10.9 | Klb 11.9 | Esh15.2 | Esh 10.9 | Klb 12.6 | Cnd 11.1 | Aci 10.9 | Enc 15.46 | Klb 13.5 | Esh 7.5 | Esh 9.6 | Aci 16 | Psd 15.5 |
| Esh 9.6 | Sta 10 | Psd12.7 | Cnd 9.4 | Enb 6.3 | Esh 10.2 | Esh 9.8 | Enb 4.348 | Enc 11.7 | Enc 7.5 | Enc 7.1 | Enc 9.3 | Aci 8.45 |
| Psd 7.8 | Esh 8.8 | Aci8.9 | Enc 6.9 | Enc 7.7 | Enc 7.4 | Enb 6.1 | Cnd 3.865 | Prt4.7 | Cnd5 | Enb 5.7 | Sta 6.6 | Cnd 7 |
| Enb 4.8 | Enc 6.9 | Sta3.8 | Sta 5.4 | Prt 4.3 | Enb 6.5 | Enc 3.6 | Aci 2.415 | Enb 4.5 | ONF 3.8 | Sta 3.8 | Prt 5.3 | Enb 4.23 |
| Oth 10.9 | Oth 11.3 | Oth8.9 | Oth 11.7 | Oth 12.3 | Oth 9.2 | Oth 13.4 | Oth 1.449 | Oth 15.6 | Oth 16.2 | Oth 11.5 | Oth 8 | Oth 11.3 |
* Aci – Acinetobacter spp, Klb – Klebsiella spp, Esh – Escherichia spp, Enb – Enterobacter spp, Psd – Pseudomonas, Prt – Proteeae tribe, Sta – Staphylococcus aureus, Enc- Enterococcus, Cnd – Candida
*Oth – Others –Aeromonas, Salmonella, Shigella, Serratia, H.influenzae, ONF - Other Non Fermenting GNB, Citrobacter, Streptococcus spp, CONS.
NICU- neonatal ICU, PICU-pediatric ICU, Ped SG ICU- pediatric surgery ICU, MICU-medicine ICU, CCU - critical care unit, NMICU- neuromedicine ICU, Onco ICU-oncology ICU, Uro ICU-urology ICU, SICU-surgery ICU, CTVS ICU-cardiothoracic and vascular surgery ICU, NSG ICU-neurosurgery ICU, PSG ICU-plastic surgery ICU, SGE ICU-surgical gastroenterology ICU. (KTP ICU did not have any relevant case during the study period).
The decreasing order of the common organisms isolated from various clinical specimens has been depicted in [Table/Fig-5]. Acinetobacter spp from tracheal aspirate and Pseudomonas spp from blood specimens were the most common organism isolated; whereas Escherichia coli was the predominant organism in urine, exudate and sterile fluid specimens.
Distribution of pathogens in various clinical isolates (Percentage).
| Tracheal aspirates (924) | Exudate (702) | Urine (602) | Blood (497) | Sterile fluid (225) |
|---|
| Acinetobacter 332(36%) | E.coli 130(18.5%) | E.coli 176(29.2%) | Pseudomonas 108(21.8%) | E.coli 54(24%) |
| Pseudomonas 255(27.6%) | Pseudomonas 109(15.5%) | Candida 130(21.6%) | Acinetobacter 82(16.5%) | Klebsiella 38(16.9%) |
| Klebsiella 147(16%) | Klebsiella 108(15.3%) | Enterococcus 98(16.3%) | Klebsiella 65(13.1%) | Acinetobacter 26(11.5%) |
| E.coli 49(5.3%) | Enterococcus 92(13.1%) | Klebsiella 72(12%) | Candida 55(11.1%) | Enterococcus 23(10.2%) |
| S.aureus 34(3.7%) | Acinetobacter 80(11.4%) | Pseudomonas 66(11%) | Enterococcus 54(10.9%) | Pseudomonas 22(9.7%) |
| Enterobacter 28(3%) | S.aureus 68(9.7%) | Enterobacte 27(4.5%) | S.aureus 37(7.4%) | Enterobacter 21(9.3%) |
| Other NF 30(3.2%) | Enterobacter 24(3.4%) | Acinetobacter 9(1.5%) | Enterobacter 34(6.9%) | Candida 10(4.4%) |
Other NF- Non fermenting Gram Negative bacilli other than Pseudomonas and Acinetobacter.
Less frequently isolated organisms were excluded in this table
Hospital Acquired Infections in ICU Patients:
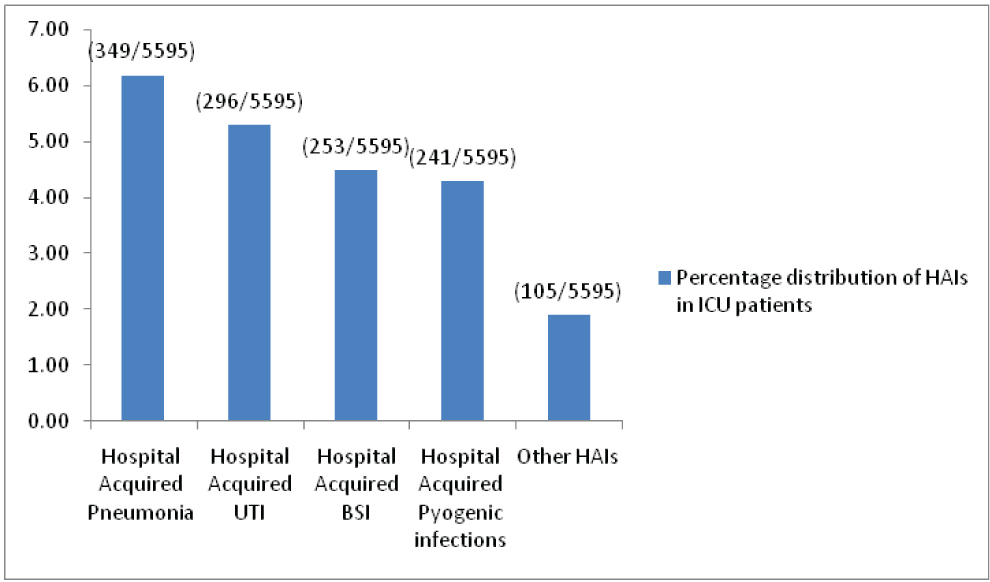
From the data obtained we also analysed HAI cases among ICU patients included in the study. We found that about 22.2% (1244 out of 5595 patients admitted to ICUs) infections are HAIs, Out of which hospital acquired pneumonia (6.24%) was the most common [Table/Fig-6]. HAI were more in MICU and NICU compared to other ICU’s; which can be explained by more ICU bed capacity in these ICU’s.
Hospital acquired infection in ICU patients. (n = 1244).
Antimicrobial Resistance Pattern of clinical isolates:
AMR pattern of non-fermenting GNB, Enterobacteriacae family GNB, and gram-positive cocci were depicted in [Table/Fig-7,8 and 9] respectively. In our study we noted that non-fermenting GNB ranks top in the list among clinical isolates from ICU. Antimicrobial susceptibility pattern was analysed among these isolates and it was observed that most of NF-GNB were multi drug resistant organisms being resistant to three or more class of antibiotics. High rates of resistance was noted to even carbapenems and aminoglycosides. Members of the Enterobacteriacae family are the second in the list of most common clinical isolates. Escherichia coli and Klebsiella spp were most common clinical isolates in this family. In both of these GNB, high rates of non susceptibility was noted against quinolones, cephalosporins and beta lactam inhibitor group of drugs. Resistance to carbapenems was also significantly higher. In the present study, 40.6% of Staphylococcus aureus were found to be MRSA and the proportion of VRE was 11.9%. Candida spp were isolated in clinical specimens (6.89%) and was noted that 5.5% of isolates were resistant to fluconazole.
Antimicrobial resistance pattern of non fermenting GNB.
| Antimicrobial agent | Acinetobacterspp (541) | Pseudomonasspp (590) | Other NF-GNB(51) |
|---|
| Ceftazidime | 490 (90.6%) | 438 (74.2%) | 34 (66.6%) |
| Cefoperazone/Sulbactam | 138 (25.5%) | 137 (23.2%) | 6 (11.8%) |
| Meropenem | 452 (83.5%) | 330 (56%) | 28 (55%) |
| Gentamycin | 472 (87.2%) | 407 (69%) | 31 (60.8%) |
| Amikacin | 457 (84.5%) | 335 (56.7%) | 21 (41.2%) |
| Ciprofloxacin | 468 (86.5%) | 394 (66.8%) | 17 (33.3%) |
| Tetracycline | 246 (45.8%) | - | - |
*% indicates resistance of a clinical isolate to corresponding antimicrobial agent
Antimicrobial resistance pattern of enterobacteriacae GNB.
| Antimicrobial agent | Escherichiacoli (457) | Klebsiella spp (456) | Enterobacter spp (143) | Proteeae tribe (74) | Citrobacter spp (27) |
|---|
| Ceftriaxone | 411 (89.9%) | 381(83.5%) | 125(87.4%) | 52(70.3%) | 21(77.8%) |
| Ceftazidime | 389(85.1%) | 377(82.7%) | 121(84.6%) | 50(67.6%) | 23(85.2%) |
| Cefoperazone/ Sulbactam | 61(13.3%) | 80(17.5%) | 26(18.2%) | 7(9.5%) | 4(14.8%) |
| Meropenem | 99(21.6%) | 204(44.7%) | 73(51.1%) | 7(9.5%) | 10(37%) |
| Gentamycin | 292(63.9%) | 311(68.2%) | 109(76.2%) | 47(63.5%) | 18(66.6%) |
| Amikacin | 109(23.9%) | 221(48.4%) | 83(58%) | 40(54.1%) | 16(59.3%) |
| Ciprofloxacin | 412(90.1%) | 358(78.5%) | 110(77%) | 57(77%) | 20(74.1%) |
*% indicates resistance of a clinical isolate to corresponding antimicrobial agen
Antimicrobial resistance pattern of gram positive cocci.
| Antimicrobialagent | Staphylococcusaureus (155) | Enterococcusspp (286) | Streptococcusspp (44) |
|---|
| Penicillin | 134(86.5%) | - | 3(6.8%) |
| Ampicillin | - | 177(61.9%) | - |
| Gentamycin | 50(32.3%) | - | - |
| Ciprofloxacin | 107(69 %) | | - |
| Erythromycin | 68(43.9%) | - | 6(13.6%) |
| Clindamycin | 23(14.8%) | - | - |
| High level Gentamycin | - | 81(28.3%) | - |
| Vancomycin | 0(0.00%) | 34(11.9%) | - |
| Tetracycline | - | 217(75.9%) | 22(50%) |
| Cefoxitin | 63(40.6%) | - | - |
*% indicates resistance of a clinical isolate to corresponding antimicrobial agent
Multidrug resistance in Gram-Negative Bacilli (Coresistance patterns):
The multidrug resistance pattern among significant clinical isolates of gram-negative bacilli has been depicted in [Table/Fig-10]. Of the 2044 GNB isolates recovered, 1139 (55.7%) isolates were MDR i.e., resistant to at least three or more classes of antimicrobial agents. About 31.3% and 13.6% of isolates were resistant to four and three classes of antimicrobial drugs respectively. It was observed that 41.2% of Acinetobacter isolates were resistant to 5 major classes of antibiotics. Significant resistance to carbapenems was noted among all classes of gram negative bacteria.
Multidrug resistance pattern among significant clinical isolates of Gram Negative Bacilli.
| Acinetobacter spp(541) | Pseudomonas spp(590) | Escherichia coli(457) | Klebsiella spp(456) | Total MDR GNB(2044) |
|---|
| 5 MDR (AMG+ CEPH + FQ+CARB+TETRA) | 223(41.2%) | - | - | - | 223** |
| 4 MDR (AMG + CEPH+ FQ + CARB) | 178(32.9%) | 199(33.7%) | 86(18.8%) | 176(38.6%) | 639(31.3 %) |
| 3 MDR (AMG + CEPH + FQ) | 19(3.5%) | 55(9.3%) | 22(4.8%) | 38(8.3%) | 134(6.6%) |
| 3 MDR (CARB + CEPH + FQ) | 12(2.2%) | 54(9.1%) | 12(2.6%) | 19(4.1%) | 97(4.7%) |
| 3 MDR (AMG + FQ + CARB) | 2(0.4%) | 15(2.5%) | 0 | 3(0.7%) | 20(1%) |
| 3 MDR (AMG + CEPH+ CARB) | 10(1.8%) | 13(2.2%) | 0 | 3(0.7%) | 26(1.3%) |
| Total MDR (%) | 444 (82.1%) | 336 (56.9%) | 120 (26.3%) | 239 (52.4%) | 1139 (55.7%) |
*AMG – Aminoglycosides (amikacin and gentamycin), CEPH – Cephalosporins (ceftriaxone and ceftazidime except for Acinetobacter and Pseudomonas where only ceftazidime is tested), FQ – Quinolones (ciprofloxacin), CARB – Carbapenems (meropenem), TETRA – Tetracyclines (tetracycline). ** Tetracycline was tested only for Acinetobacter
Discussion
AMR is an increasingly threatening emerging problem in majority of health care facilities. Multi-drug resistant HAI are one of the major causes of deaths and morbidity amongst inpatients of hospital. It is known that ICU is an epicenter of HAI [3,18]. Hence this study was undertaken to determine the microbial versatility and non-susceptibility of these organism to commonly prescribed antimicrobial agents in a tertiary care hospital.
The demographic parameters of the ICU patients in this study revealed that the number of males admitted in the ICU was almost double to that of female, and the mean age of patients was around 47 years. This finding is concordant with study conducted by Nikhilesh Anand et al., Mahendra K Patel et al., [19,20].
In spite of efficient disinfection procedures for the respiratory equipment, hospital acquired bacterial pneumonia continues to be the major cause of HAIs in ICU setting and their frequency vary from 7-41% of ICU patients who are on continuous mechanical ventilation. This was emphasized in our study with the most common clinical specimen received being tracheal aspirate (29.9%). Similar findings have also been derived by Mahin Jamshidi et al., in a study conducted in Infectious Disease Research Center, Iran [21]; Sugata Dasgupta et al., conducted in ICU in a tertiary care set up of eastern India [22].
As a result of irrational use of antimicrobials, non-fermenting gram-negative bacilli (NF-GNB) have emerged as important hospital acquired pathogens. These pathogens are inhabitant of nature particularly in soil and water. In the hospital environment, they may be isolated from instruments such as ventilators, hospital linens as well as from the skin of HCWs [23]. In the present study also, NF-GNB were the most common group of organisms isolated from the ICUs across JIPMER.
In our study, the most common organism isolated from ICU was Pseudomonas spp (19.1%) followed by Acinetobacter spp (17.5%). Similar findings were reported by Kiran Chawla et al., in a retrospective study conducted at a tertiary care hospital [24], where NF-GNB was the most common pathogen isolated. Jean-Louis Vincent et al., reported that Pseudomonas spp was increasingly associated with infection in health care settings [25]. Javeri Jitendra R et al., also reported that Acinetobacter spp as the second most common isolate in ICU of tertiary care center [26]. Other NF-GNB are isolated less frequently (<1%) which include Burkholderia spp, Elizabethkingia meningoseptica and Shewanella spp. Most of NF-GNB isolated were found to be multidrug resistant and hence they pose challenge to the treating physician.
Next to NF-GNB, members of Enterobacteriaceae GNB (EB-GNB) such as Escherichia coli (14.8%) followed by Klebsiella spp (14.7%) were the next common group isolated in the clinical specimen. They were increasingly reported from urine and exudate specimen in contrast to NF-GNB such as Pseudomonas spp and Acinetobacter spp which were the most common organism isolated from tracheal aspirate. This is because of increased colonization of NF-GNB in the respiratory tract of patients on prolonged mechanical ventilation.
As a novel initiative, we also analysed the most common clinical isolate in each ICU [Table/Fig-5]. We found that Klebsiella spp, Escherichia coli and Acinetobacter spp were higher among the various paediatric ICUs. In adult ICUs, Pseudomonas spp, Klebsiella spp, Acinetobacter spp and Escherichia coli were increasingly isolated.
We also studied the distribution of pathogens in various clinical isolates and found that Acinetobacter spp and Pseudomonas spp are the most common pathogen isolated from tracheal aspirate and blood respectively; whereas E.coli is most commonly isolated from exudate, urine and sterile fluids. Various study conducted by Mohammadi-mehr M et al., Maksum Radji et al., Azizun Nahar et al., Kaushal V Sheth et al., Mahin Jamshidi et al., [27–30] also derived similar findings [21]. As repeated blood specimens were not sent from all the patients, hence the significance of NF-GNB such as Pseudomonas and Acinetobacter as pathogen or contaminant cannot be commented.
About 22.2% of patients admitted to ICUs developed HAIs. Pneumonia accounted for the most common HAIs in ICU setting followed nosocomial UTI and blood stream infections. Most studies published elsewhere considers pneumonia as the second most common nosocomial infection in ICUs next to UTI [9,25]. However, it may account for the most common HAI in mechanically ventilated patients. As most ICU patients in our studies are critically ill and mechanically ventilated, this explains why pneumonia was found to be most common HAI in the ICUs in our study.
Antimicrobial Resistance Pattern:
AMR is an emerging clinical problem in ICUs including neonatal, paediatric and various adult critical care units. Resistance of NF-GNB has emerged widely, and also multidrug resistance and pan drug resistance have been reported by many studies causing real challenge to the treating ICU intensivists. AMR is also widely prevalent among Enterobacteriaceae GNB (EB-GNB) and gram-positive organisms. There are several studies which report MDR NF-GNB, MDR Enterobacteriacae, MRSA, VISA, VRE in ICU patients [17,21,23]. In our study we analysed antimicrobial non-susceptibility pattern under three categories such as NF-GNB, EB-GNB, and gram-positive organisms.
Among the NF-GNB isolates, a high degree of resistance has been observed to almost all classes of antimicrobials tested such as cephalosporins, carbapenems, aminoglycosides and quinolones. This data is supported by various studies Mohammadi-mehr M et al., Maksum Radji et al., Kaushal V Sheth et al., [27,28,30]. Acinetobacter spp was the most resistant organism in our study. A 83.5% of Acinetobacter spp were found to be resistant to be meropenem; whereas the cephalosporin resistance varied between 90.6% (ceftadizime) and 25.5% (Cefoperazone/ Sulbactam). Even resistance to aminoglycosides and quinolones were also much higher (84.5% to amikacin and 86.5% to ciprofloxacin). Kaushal V Sheth et al., had reported concordance resistance pattern for Acinetobacter spp to various class of antimicrobials [30]. Tetracycline was found to be more effective against Acinentobacter (45.8%) compared to the other antimicrobials, hence it may be a promising agent for the treatment of Acinentobacter infections. Pseudomonas species were also found to be resistant to several classes of antimicrobials tested, but the intensity of resistance was lower compared to Acinetobacter spp. Resistance of isolates of Pseudomonas to cefoperazone/ sulbactam, meropenem and amikacin was found to be 23.2%, 56% and 56.7% respectively whereas ciprofloxacin resistance was around 66.8%. This is also in agreement to several other Indian studies published in the recent past [27,28,30].
Acinetobacter spp and Pseudomonas spp are the major cause of infections in ICU patients [23]. They are often found to be colonized on patient’s respiratory tract. Being multidrug resistant, they flourish in the respiratory tract of ICU patients who are often on multiple antimicrobials. Presence of other comorbid conditions such as unconsciousness, endotracheal tube insertion, prolonged ventilation pave the way for aspiration of the colonized organisms to lower respiratory tract.
Among EB-GNB, we noted high degree of resistance to cephalosporins (67.6%-89.9%) & quinolones (74.1%-90.1%) and aminoglycosides (48.2%-76.2%). Resistance was lower on addition of beta lactamase inhibitor (14.8%-18.2 % resistance against cefoperazone/sulbactam). Significant resistance to carbapenems (9.5%-51.1%) was also seen. In general, Klebsiella species followed by Enterobacter species were found to be more resistant as compared to Escherichia coli. This data is supported by studies of Mahin Jamshidi et al., Mohammadi-mehr M et al., Ganguli NK et al., [21,27,30].
In the gram-positive group, a higher degree of resistance of S.aureus was found to be against penicillin (86.5%) followed by ciprofloxacin (69%). This finding is also supported by Maksum Radji et al., Kaushal V Sheth et al., Ganguli NK et al., [28,30,31]. In our study, about (40.6%) of S.aureus were found to be MRSA (resistance to cefoxitin). There are several studies which documented the incidence of MRSA between (30%-50%) among hospitalized patients [8,28,30]. The increasing incidence of MRSA is alarming as no beta lactams drug would work in this situation and increase use of vancomycin opens the possibility of emergence of vancomycin resistance in S.aureus in near future. However, no vancomycin resistance has been reported in our study in concordance to other Indian studies.
In the present study, the Enterococci isolates were found to be resistant to ampicillin (61.9%), tetracycline (75.9%) and high level gentamycin (28.3%). Presence of high-level aminoglycoside resistance in Enterococci eliminates the synergistic bactericidal effect of beta lactam agent with an aminoglycoside which is usually recommended for the treatment of serious enterococcal infections [32]. VRE has been observed in 11.9% (34 out of 286) of isolates in our study; E.faecalis accounts for 4.9% and E.faecium 17.5%. VRE has been an emerging problem in hospital settings which is comparatively lower as compared various western studies which reported VRE up to 33-40% (in European studies) and as high as 50% (in American studies) [32,33]. As with MRSA, the major source of VRE in hospitals is cross-contamination between patients through HCWs and admission of already colonized patients to the ICU. However, in contrast to the spread MRSA which is mainly chromosomally mediated (hence no interspecies transfer), VRE genes can be transmitted to other Enterococci species through plasmids [32,33].
The prevalence of MDR among gram-negative bacilli has significantly increased in recent times [17,28]. Emergence of these organisms can lead to increased mortality, morbidity, economic burden and longer hospital stay. We made an attempt to calculate coresistance among MDR gram-negative organisms. We found that 55.7% (1139 out of 2044) of the significant GNB isolates were MDR and are resistant to at least 3 or more classes of antimicrobials. About 31.3% and 13.6% of isolates were resistant to four and three classes antimicrobial drugs respectively. Maximum MDR was reported from Acinetobacter spp (82.1%), followed by Pseudomonas spp (56.9%), Klebsiella spp (52.4%) and Escherichia coli (26.3%). This was supported by study conducted by Aurora E et al., where it was found that 35% of GNB isolates were resistant to 4 antimicrobial groups, and 12% were resistant to 5 antimicrobial groups [17,28].
Limitation
There are several limitations of this study that needs to be addressed. First, as it is a retrospective study, adequate data on clinical information is lacking. Hence, difference between a pathogen and a contaminant is difficult to obtain especially when it comes to isolation of Pseudomonas and Acinetobacter from blood. Second, the study does not look for outcome of the patients with hospital acquired infections. Third, we have also not analysed the choice of treatment given to the patients with infection associated with MDR organisms. Fourth, the definition of MDR-GNB used in this study was based on clinical practices and focused on commonly prescribed antimicrobials. At present, a standardized definition for MDR-GNB is lacking [17]. Different definitions may produce different resistance pattern.
Conclusion
HAI and AMR in the ICUs are the major obstacles to patient’s outcome; may pose several detrimental effect in terms of increase in length of hospital stay, financial burden and mortality rate. Reduction in both HAIs and AMR is the most difficult challenge as well as the goal for the hospital administrators around the world. The increasing trend of MDR-GNBs especially to higher generation cephalosporins and carbapenems is most worrisome problem as it leaves with no other antimicrobial treatment options except colistin. Similarly increase spread of MRSA and VRE pose a great threat to HCWs as well as to the other critically ill patients of the ICUs.
There is also lack of data on antimicrobial surveillance. Study on AMR surveillance is the need of the hour as it helps the centers to generate local antibiogram which further helps in having a national data. It also guides the clinicians to choose appropriate empirical therapy and assist escalation and de-escalation wherever possible. Hence, such studies will be a stepping stone in establishing antimicrobial stewardship and regulate the antimicrobial use. A robust and effective hospital infection control policy, antimicrobial stewardship programme with frequent revisions of antimicrobial policy guideline is mandatory and is the only way to control HAIs and AMR.