Paraneoplastic Glomerulopathy in a Case of Collecting Duct Renal Cell Carcinoma
Srikanth Prasad Devarsetty1, Dharshan Rangaswamy2, Shailaja Bhat3, Shankar Prasad Nagaraju4, Ravindra Prabhu Attur5
1 Resident, Department of Nephrology, Kasturba Hospital, Manipal University, Manipal, Karnataka, India.
2 Associate Professor, Department of Nephrology, Kasturba Hospital, Manipal University, Manipal, Karnataka, India.
3 Associate Professor, Department of Pathology, Manipal University, Manipal, Karnataka, India.
4 Associate Professor, Department of Nephrology, Kasturba Hospital, Manipal University, Manipal, Karnataka, India.
5 Professor and Head, Department of Nephrology, Kasturba Hospital, Manipal University, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Dharshan Rangaswamy, Department of Nephrology, Kasturba Hospital, Manipal University, Madhav Nagar, Manipal- 576104, Karnataka, India.
E-mail: doctor.dharshan@gmail.com
Paraneoplastic glomerulopathy has been described in established cases of the solid tumors of lung, gastrointestinal system, breast, etc., and rarely in patients with Renal Cell Carcinoma (RCC). Studies on secondary glomerular diseases have described a higher incidence of IgA nephropathy in patients with RCC compared to membranous glomerulopathy, which are commonly reported in malignancies of the lung and gastrointestinal tract. Collecting Duct Carcinoma (CDC), a rare high grade adenocarcinoma accounts for <1% of all renal malignancies. It arises from the cells of the collecting ducts of Bellini. We report a case of an elderly male who was diagnosed to have a disseminated CDC during his evaluation for nephrotic syndrome. Renal biopsy was suggestive of a secondary membranous glomerulonephropathy.
Immunohistochemistry, Malignancy, Nephrotic syndrome
Case Report
A 65-year-old male, diabetic for the last two years and hypertensive since last six months presented with gradual onset of progressive pedal edema and facial puffiness for the last one month. This was associated with frothy urine and was not associated with gross hematuria or obstructive lower urinary tract symptoms. He had no past history of edematous illness, joint pain, rash, jaundice, drug abuse or alternative medicine intake. On physical examination he was normotensive with mild pallor, bilateral pitting pedal edema and fundus examination showed no evidence for diabetic or hypertensive retinopathy. On further evaluation, he had normal renal function and thyroid profile, a 24hr urine protein of 4.8gm with a serum albumin of 1.80g/dL, microscopic hematuria and an elevated serum complements. Auto-immune work up, including Anti-Nuclear Antibodies (ANA) and Anti-Phospholipase A2 Receptor (APLA2R) antibodies was negative. His cardiac evaluation and echocardiogram was reported as normal. Ultrasonography of the abdomen revealed moderate right sided hydroureteronephrosis.
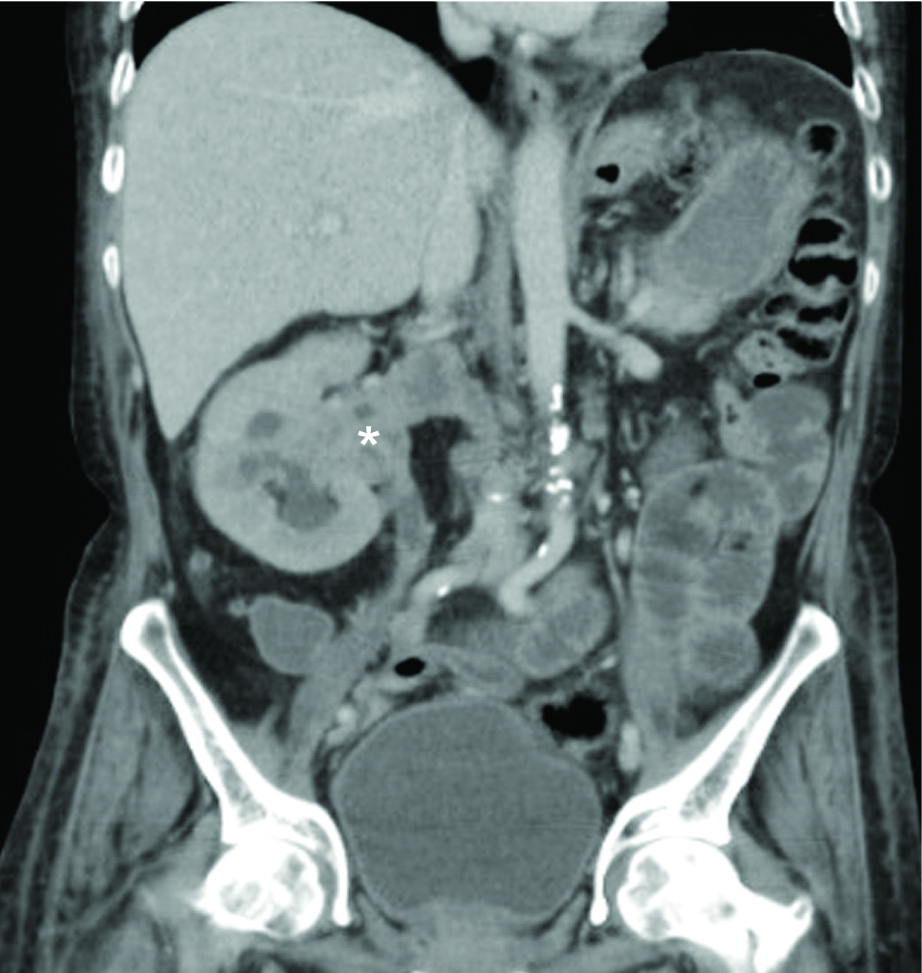
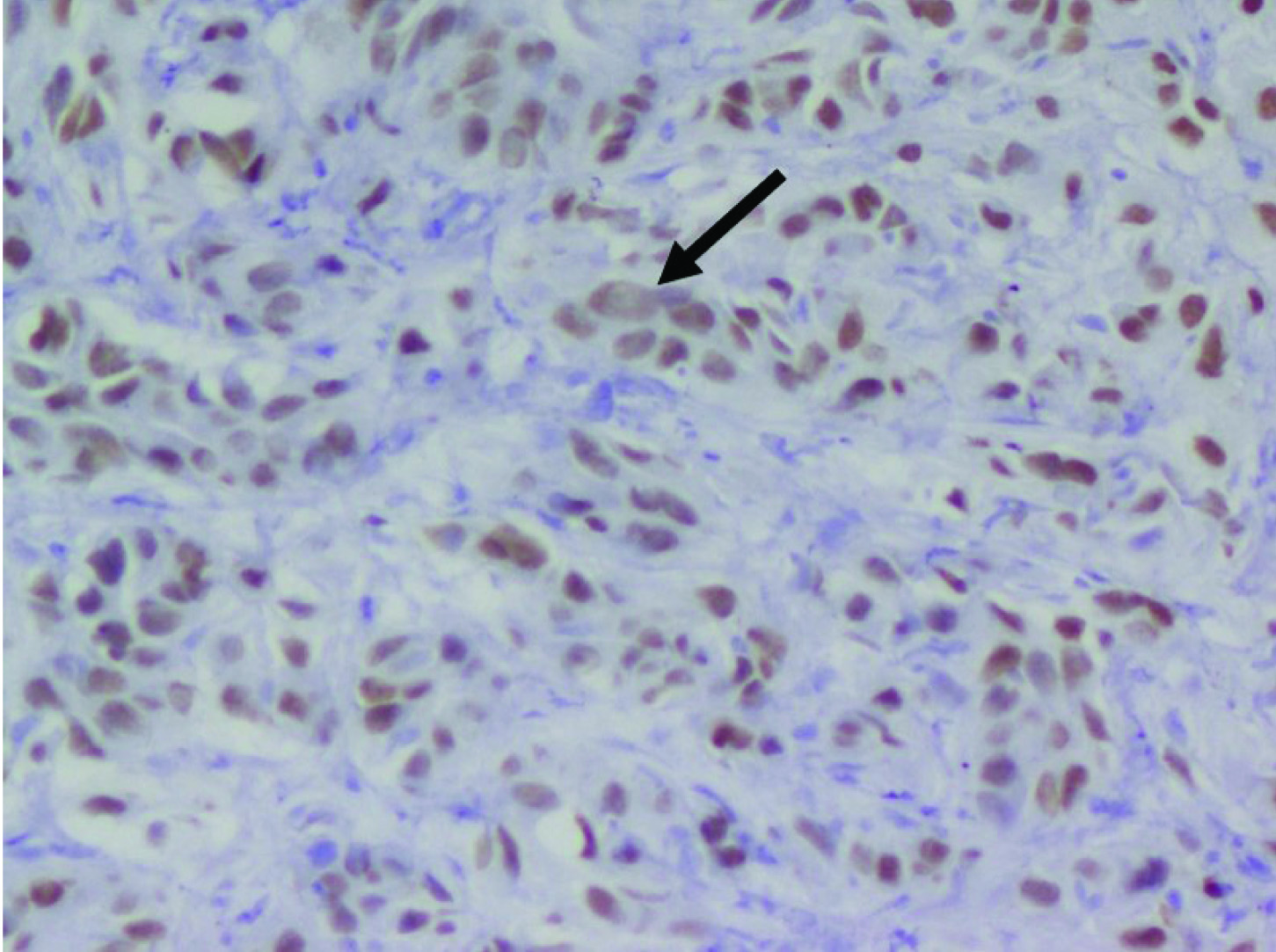
Contrast Enhanced Computed Tomography (CECT) of the abdomen showed a heterogeneously hypo-enhancing soft tissue lesion at the pelvis of right kidney [Table/Fig-1] with malignant para-aortic lymph nodes and a possible left adrenal gland metastasis. Computed Tomography (CT) guided biopsy from the lesion revealed an infiltrating tumor composed of spindle shaped cells with large hyperchromatic pleomorphic nuclei and moderate amount of eosinophilic cytoplasm (high N/C ratio) surrounded by desmoplastic stroma [Table/Fig-2]. Immunohistochemistry (IHC) showed the tumor cells stained positively for Paired box gene 8 (PAX8) and negative for Renal Cell Carcinoma (RCC), Cytokeratins 7 (CK7), Cytokeratin 20 (CK20) and Protein 63 (p63). Hemoglobin electrophoresis showed a normal adult pattern. Diagnosis of Collecting Duct Carcinoma (CDC) was established based on clinical setting, histology and IHC. A percutaneous left renal biopsy was done which was reported as Membranous Nephropathy (MGN) with immunofluorescence positive for IgG1 and negative for IgG4 staining. With the clinical background of an elderly male presenting with Nephrotic Syndrome (NS), a negative immune workup including APLA2R antibodies and renal biopsy suggestive of MGN with absent immunofluorescence staining for IgG4, a diagnosis of disseminated malignancy with secondary MGN was concluded. The nature of the disease and therapeutic options were discussed. Patient and his relatives wished for conservative approach without any definitive surgical or anticancer therapy. Patient was initiated on angiotensin converting enzyme inhibitors with statins and at last follow up, patient continued to have features of NS.
Coronal section of contrast enhanced computed tomography (CECT) of the abdomen showing a heterogeneously hypo-enhancing soft tissue lesion at the pelvis of right kidney (*) extending into the right ureter, calyces, right renal vein and inferior vena cava.
Tumor cells showing intense nuclearpositivity for PAX8 (thick arrow) with desmoplastic stroma (Magnification 40X; PAX8).
Discussion
Diagnosis of RCC in a patient who presents primarily with NS is a rare clinical event. RCC is histologically divided into: clear cell RCC, papillary RCC, chromophobe RCC, multilocular cystic RCC, CDC, medullary carcinoma, mucinous tubular and spindle cell carcinoma, neuroblastoma associated. RCC, Xp11.2 translocation–TFE3 carcinoma, and unclassified lesions [1]. CDC is a rare tumor accounting for <1% of all renal malignancies [2]. It arises from the cells of the collecting ducts of Bellini. CDC is primarily a high-grade adenocarcinoma with glandular architecture and desmoplastic stroma occurring predominantly in older adults. It is an extremely aggressive tumor, with most patients presenting with metastatic disease. The most common clinical presentation is hematuria. Proteinuria especially sub-nephrotic may be seen in association with RCC but NS is only rarely seen; often as part of paraneoplastic glomerulopathy. The other paraneoplastic manifestations which may be seen in upto 10%-40% of patients with RCC are hypercalcemia, hypertension, polycythemia, impaired glucose tolerance, Cushing’s syndrome, thrombosis and amyloidosis [3]. Paraneoplastic glomerulopathy was the term initially described in 1922 by Galloway in a patient of NS in association with Hodgkin’s disease [4]. The most common paraneoplastic glomerulopathy is MGN often seen in association with gastrointestinal and lung malignancies [5]. The occurrence of glomerular diseases in RCC is rare. Various lesions such as MGN, IgA nephropathy, mesangiocapillary glomerulonephritis, crescentic glomerulonephritis and amyloidosis have been reported. In a large series of patients with RCC and paraneoplastic glomerulopathy, IgA nephropathy has been more common than MGN. The infiltrating plasma cells around the RCC produced IL-6, which would then increase the level of circulating IgA, which deposits in the mesangial area causing IgA nephropathy [6]. The pathological features of malignancy associated MGN are similar to MGN due to other secondary causes and APLA2R antibodies are typically negative. Also, the absence of IgG4 staining on renal biopsy favors the possibility of secondary MGN in our patient. The mechanism of occurrence of MGN in RCC has been proposed to be due to an antibody response to the tumor associated antigens deposited in the glomeruli and formation of tumor antigen-antibody immune complexes. Another thought is that patients with RCC are rendered more susceptible to the immune complex injury caused by either exogenous or endogenous antigens secondary to alterations in their immune function [7]. Because of the rarity in incidence of CDC, occurrence of paraneoplastic glomerulopathy in patients with CDC is not well recognized. Our patient had a tumor involving the renal pelvis. Differential diagnosis of high-grade carcinomas involving the renal sinus includes Urothelial Carcinoma (UC) of the upper urinary tract, CDC, Renal Medullary Carcinoma (RMC), Clear cell type RCC and Papillary RCC (especially type 2). Differentiating UC from CDC and RMC is often difficult, especially with small biopsy samples due to significant morphologic overlap between these tumors. Immunohistochemistry is often utilized to make this distinction. Our patient had an immunoprofile of PAX8+/ RCC-/p63-/ CK7-/ CK20-. The RCC marker is a monoclonal antibody against a glycoprotein in the brush border of proximal tubular cells [8]. It is positive in almost all papillary and clear cell RCCs but is typically negative in CDC, RMC and UC [9]. Tumor protein p63 is normally expressed in the basal cell layers of the urothelium but not in the kidney [10]. The p63 is expressed in up to 70%–100% of patients with UC [11]. PAX8 is a nuclear transcription factor that is expressed normally in the podocytes and cells of the distal tubule; but may also be expressed by other parts of the nephron when they are damaged. It has both cytoplasmic and nuclear staining and when positive, it stains a high percentage of tumor cells [12]. Albadine et al., proposed that the immunoprofile of PAX8+/p63− supports the diagnosis of CDC with a sensitivity of 85.7% and a specificity of 100%. In contrast, a PAX8−/p63+ profile supports the diagnosis of UC with a sensitivity of 88.2% and a specificity of 100% [11]. CDC and RMC share a common immunoprofile but RMC has been reported to occur almost exclusively in young patients with sickle cell trait or disease [13]. The treatment of choice for paraneoplastic glomerulopathy secondary to RCC is surgical removal of the tumor. Complete or partial remission of the NS has been reported in some cases following surgical intervention [14]. Steroids which form the backbone of immunosuppression for management of primary NS is controversial and may lead to cyst growth as was seen in a patient treated with prednisolone for secondary IgA nephropathy [15]. Thus, steroid use must be limited to only those cases with persistent NS after the RCC has been controlled.
Conclusion
Paraneoplastic glomerulopathy is a rare occurrence. Membranous glomerulonephritis associated with gastrointestinal and lung malignancies and IgA nephropathy in the setting of RCC are occasionally described. Diagnosis of secondary glomerular disease is important for prognostication and management; therapy mainly involves targeting the primary malignancy. IHC is essential for accurate diagnosis and sub-tying of RCC. An immunoprofile of PAX8+/p63− supports the diagnosis of CDC in the appropriate clinical setting.
[1]. Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlationRadiographics 2006 26:1795-806. [Google Scholar]
[2]. Cohen HT, McGovern FJ, Renal-cell carcinomaN Engl J Med 2005 8:2477-90. [Google Scholar]
[3]. Palapattu GS, Kristo B, Rajfer J, Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinomaRev Urol 2002 4:163-70. [Google Scholar]
[4]. Galloway J, Remarks on Hodgkin’s diseaseBr Med J 1922 23:1201-08. [Google Scholar]
[5]. Bacchetta J, Juillard L, Cochat P, Droz JP, Paraneoplastic glomerular diseases and malignanciesCrit Rev Oncol Hematol 2009 70:39-58. [Google Scholar]
[6]. Akihiro Tojo (2013). ParaneoplasticGlomerulopathy Associated with Renal Cell Carcinoma, Renal Tumor, Prof. Jindong Chen (Ed.), ISBN: 978-953-51-0981-5, InTech, Available from: http://www.intechopen.com/books/renal-tumor/paraneoplastic-glomerulopathy-associated-with-renal-cell-carcinoma [Google Scholar]
[7]. Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, A mechanism for cancer-associated membranous nephropathyN Engl J Med 2016 374:1995-96. [Google Scholar]
[8]. McGregor DK, Khurana KK, Cao C, Tsao CC, Ayala G, Krishnan B, Diagnosing primary and metastatic renal cell carcinoma: the use of the monoclonal antibody ‘Renal Cell Carcinoma marker’Am J SurgPathol 2001 25:1485-92. [Google Scholar]
[9]. Bakshi N, Kunju LP, Giordano T, Shah RB, Expression of renal cell carcinoma antigen (RCC) in renal epithelial and non renal tumors: diagnostic implicationsAppl Immunohistochem Mol Morphol 2007 15:310-15. [Google Scholar]
[10]. Tuna B, Unlu M, Aslan G, Secil M, Yorukoglu K, Diagnostic and prognostic impact of p63 immunoreactivity in renal malignanciesAnal Quant Cytol Histol 2009 31:118-22. [Google Scholar]
[11]. Albadine R, Schultz L, Illei P, Ertoy D, Hicks J, Sharma R, PAX8 (+)/p63 (-) immunostaining pattern in renal collecting duct carcinoma (CDC): a useful immunoprofile in the differential diagnosis of CDC versus urothelial carcinoma of upper urinary tractAm J Surg Pathol 2010 34:965-69. [Google Scholar]
[12]. Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical studyMod Pathol 2009 22:1218-27. [Google Scholar]
[13]. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs 2004 Lyon, FranceIARC PressWorld Health Organization Classification of Tumors; vol 7 [Google Scholar]
[14]. Kuroda I, Ueno M, Okada H, Shimada S, Akita M, Tsukamoto T, Nephrotic syndrome as a result of membranous nephropathy caused by renal cell carcinomaInt J Urol 2004 11:235-38. [Google Scholar]
[15]. Mimura I, Tojo A, Kinugasa S, Uozaki H, Fujita T, Renal cell carcinoma in association with IgA nephropathy in the elderlyAm J Med Sci 2009 338:431-32. [Google Scholar]