Obesity is a worldwide epidemic. It is one of the leading preventable cause of death worldwide with increasing prevalence in both adults and children.
Various options are available for the treatment of obesity broadly classified into non-surgical management and surgical interventions. Currently, surgical procedures are the most effective therapy for long-term weight loss in morbidly obese and severely obese individuals with comorbidities. As per International Federation for the Surgery of Obesity and Metabolic disorders-Asia Pacific Chapter (IFSO-APC) consensus guidelines, bariatric surgery should be considered for the treatment of obesity for acceptable Asian candidates with BMI ≥ 35 with or without comorbidities as well as for the treatment of T2DM or metabolic syndrome for patients with BMI ≥ 30 who are inadequately controlled by lifestyle alterations and medical treatment. Bariatric surgery should also be considered as a non-primary alternative to treat inadequately controlled T2DM or metabolic syndrome, for suitable Asian candidates with BMI ≥ 27.5 [1].
Weight loss following LSG is achieved by both restriction and hormonal modulation. Firstly, reduction in stomach size with the sleeve resection restricts distention and increases the patient’s sensation of fullness (decreasing meal portion size). This restriction is further facilitated by the natural band effect of the intact pylorus which is maintained during sleeve gastrectomy. Secondly, early evidence suggests a reduction in the hunger drive of patients undergoing sleeve gastrectomy. This may be related to decreasing serum levels of ghrelin, a hormone produced mainly by P/D sub 1 cells lining the fundus of the human stomach which stimulates hunger [3–5].
Various clinical trials have shown better long-term survival following bariatric surgery than medical management for the treatment of morbid obesity. Furthermore, some of these operations lead to the rapid remission of T2DM and metabolic syndrome in a weight loss independent manner [6].
Metabolic control can be achieved with gastric restrictive procedures such as vertical banded gastroplasty, adjustable gastric banding and, more recently, LSG. However, previous studies have found that glucose homeostasis is affected by various intestinal mechanisms that are only altered by bariatric surgery procedures that include an intestinal element, such as Roux-en-Y Gastric Bypass (RYGB). A systematic review showed resolution of T2DM in 76.8% of patients undergoing RYGB and improvement of glycaemic control in 86% of patients [7]. Of the criteria used to diagnose the metabolic syndrome, fasting glucose levels are the first to return to normal in patients who have undergone sleeve gastrectomy. The achievement of normoglycaemia after bariatric procedures results from multiple changes that occur postoperatively, such as dietary control, decreased plasma ghrelin levels, weight loss, reduction of body fat, and the release of gastrointestinal hormones that interfere with the function of pancreatic β cells (incretins). An important effect of the weight loss is the improved metabolism of glucose and the reduction of insulin resistance and thus the clinical improvement of T2DM.
Owing to its merits, bariatric surgery has shown an exponential rise as a treatment modality for morbid obesity and uncontrolled T2DM in Indian population [8]. LSG has become a favoured surgical option in most of the countries owing to its cost, safety and effectiveness in weight reduction.
The aim of our study was to evaluate the effects of LSG on metabolic syndrome and central obesity in morbidly obese/severely obese Indian adults.
Material and Methods
This prospective observational study was conducted at Dayanand Medical College and Hospital, Ludhiana, Punjab, India, from August 2013 to June 2015. Based on the results observed in the existing literature and with 95% confidence and 80% power, minimum sample size came out to be 82. Therefore, a total of 100 morbidly obese (BMI>40 kg/m2) and severely obese (BMI>35 kg/m2) individuals who were suffering from diabetes, hypertension, dyslipidemia or any one of these diseases were enrolled in the study. Five patients withdrew the consent (refusal to get serial blood glycosylated haemoglobin and lipid profile levels) and four lost to follow up, thus reducing the effective sample size to 91 patients. These patients were followed up for six months and the trends of glycaemic control, mean blood pressure, lipid profile and weight loss parameters were studied. Patients with past history of any bariatric surgical intervention and patients suffering from terminal illness unlikely to be improved with weight reduction, including advanced stage cancer and end-stage renal, hepatic, and cardiopulmonary disease were excluded from the study. Diabetes mellitus was defined as serum glycosylated haemoglobin levels of more than 6.5 [9]. Hypertension was defined as systolic blood pressure of more than 160 mmHg or diastolic blood pressure of more than 90 mmHg [10]. Dyslipidemia was defined by deranged serum cholesterol or abnormally elevated Low Density Lipoprotein (LDL) or Very Low Density Lipoprotein (VLDL) [11].
Statistical Analysis
Data was analyzed using SPSS software® version 18.0. Student’s t-test and chi-square tests were used for statistical analysis.
Results
Demographic Data:
Out of 91 patients, 54 (59.34%) were males and 37(40.66%) patients were females. Mean age of patients was 42.57 years. Mean BMI was 51.27 kg/m2 [Table/Fig-1].
Patient demographics (preoperative data).
| Number (n) | 91 |
|---|
| Male | 54(59.34%) |
| Female | 37(40.66%) |
| Mean age (in years) | 42.57 |
| Diabetes (irrespective of hypertension or dyslipidemia) | 31 (36.9%) |
| Hypertension (irrespective of diabetes or dyslipidemia) | 66 (72.53%) |
| Dyslipidemia (irrespective of diabetes or hypertension) | 51 (60.71%) |
| Mean BMI (kg/m2) | 51.27 |
Effect on Weight Loss Parameters:
Body mass index and percentage Excess Weight Loss (%EWL) showed a significant reduction at three months which was sustained through postoperative six months [Table/Fig-2].
Mean weight of study population preoperative, at postoperative one week, three months and six months.
| Time | Pre-op | One week | Three months | Six months |
|---|
| Weight (kg) | 133.37±16.10 | 129.47±15.73(p=0.71) | 113.03±13.44(p<0.05) | 90.00±11.61(p<0.05) |
| Weight loss (kg) | | 3.90±1.44(p=0.21) | 20.33±4.95(p<0.05) | 43.37±10.30(p<0.05) |
| Mean % Excess weight loss | | 5.82±2.29(p=0.52) | 29.7±4.58(p<0.05) | 63.25±8.10(p<0.05) |
| Body Mass Index (kg/m2) | 51.27±4.79 | 49.76±4.66(p=0.47) | 43.44±3.56(p<0.05) | 34.52±2.85(p<0.05) |
Chi-square test applied, Pre-op- Preoperative
Effect on Type-2 Diabetes Mellitus:
There was significant reduction in mean glycosylated haemoglobin (HbA1C) levels at three and six months post-LSG. Total of 31 (36.9%) patients out of 91 were found to have T2DM, of whom, 22 patients (70.96% of diabetics) were on Oral Hypoglycaemic Agents (OHA’s). Out of these 22 patients, 13 (59.09%) patients discontinued their medications after achieving good glycaemic control. Amongst the nine patients who were on insulin, all of them (100%) had either dose reduction to less than one third of preoperative dose or switched over to OHA’s for adequate glycaemic control [Table/Fig-3].
Mean HbA1C levels preoperative and postoperative one month, three months and six months in diabetic population.
| Time | Pre-op | One week | Three months | Six months |
|---|
| HbA1C | Diabetic(n=31, 36.9%) | 7.98±1.06 | 7.63±1.15(p=0.81) | 6.53±1.03(p<0.05) | 5.51±0.76(p<0.05) |
Chi-square test applied, Pre-op- Preoperative
Effect on Hypertension:
A total of 66 (72.53%) out of 91 patients had hypertension. There was significant reduction of systolic blood pressures at three and six months postsurgery amongst hypertensive individuals. However, there was no significant change in mean diastolic blood pressures on follow up [Table/Fig-4].
Effect on mean systolic and diastolic blood pressure.
| Time | Pre-op | One week | Three month | Six month |
|---|
| Hypertension(n=66) | Systolic | 164.75±13.44 | 157.00±14.08p=0.345 | 145.38±11.14p<0.05 | 135.75±8.06P<0.05 |
| Diastolic | 82.29±2.05 | 82.57±2.40p=0.985 | 80.86±1.51p=0.298 | 80.71±1.68p=0.220 |
Chi-square test applied, Pre-op- Preoperative
Effect on Lipid Profile:
Amongst 51 dyslipidemic patients, total serum cholesterol and serum triglyceride levels decreased significantly from preoperative value at three and six months postoperatively with most of the patients showing resolution of dyslipidemia (serum cholesterol <240 mg/dl) at the end of six months. Serum LDL and serum VLDL levels also showed a significant fall at six months but at one week and three months postoperatively, the decrement was statistically insignificant. Serum HDL levels also revealed a significant fall at three and six months postsurgery [Table/Fig-5].
| Lipid Parameters | Pre-op | One week | Three months | Six months |
|---|
| Total cholesterol (mg/dl) | 274.93±51.99 | 261.10±50.45 (p=0.611) | 219.30±40.76 (p=0.00) | 176.27±26.85 (p=0.00) |
| Serum Triglycerides (mg/dl) | 309.13±81.60 | 292.77±76.15 (p=0.811) | 238.87±72.60 (p=0.001) | 189.90±51.22 (p=0.00) |
| Serum LDL (mg/dl) | 107.43±34.97 | 101.83±32.44 (p=0.896) | 90.70±28.05 (p=0.161) | 80.67±27.65 (p=0.006) |
| Serum HDL (mg/dl) | 24.60±5.02 | 28.07±5.13 (p=0.143) | 37.93±6.76 (p=0.000) | 41.50±7.63 (p=0.00) |
| Serum VLDL (mg/dl) | 38.33±13.00 | 36.97±11.99 (p=0.964) | 32.73±10.05 (p=0.210) | 29.57±8.75 (p=0.014) |
Pre-op- Preoperative
Effect on Central Obesity:
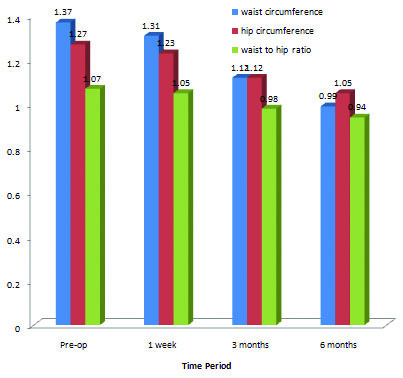
The waist and hip circumference showed a significant fall at three months and six months following LSG. However, at one week postoperatively, the decrease in waist and hip circumference was statistically insignificant when compared to the preoperative values. There was a statistically significant fall in waist to hip ratio at three and six months following LSG indicating a decrease in central obesity. The hip circumferences of patients also showed a significant fall at three months following LSG which remained at low values till six months of surgery. [Table/Fig-6].
Effect on anthropometric parameters/central obesity.
(Chi-square test)
Discussion
LSG is fast becoming a well accepted primary weight loss surgery because of its excellent weight loss results, simplicity in performing the procedure and less rate of complications. Bariatric surgery results in marked and long-lasting weight loss and elimination or improvement of most obesity-related medical complications, including diabetes, hypertension, hyperlipidemia, metabolic syndrome and obstructive sleep apnea.
The most important and prime benefit of LSG is reduction in body weight. Infact, it is an important parameter by which success or failure of any weight reducing technique is measured. In our study, mean weight loss following LSG at one week, three months and six months was 3.90 kg, 20.33 kg and 43.37 kg respectively. There was a significant decrease in BMI at three and six months following LSG in our study group with mean BMI at one week, three months and six months being 49.76 kg/m2, 43.44 kg/m2 and 34.52 kg/m2 as compared to the preoperative values of 51.27 kg/m2.
Gluck B et al., showed results similar to our study by showing mean %EWL as 49.9%, 64.2%, 67.9% at three, six and 12 months respectively after LSG [12]. Our results are also in accordance with the study conducted by Boza C et al., on 1000 patients undergoing LSG who showed as excess weight loss of 86.6% at one year, 84.1% at two years and 84.5% at three years [13].
Various factors play role in causing weight loss following LSG. These factors may be related to the restriction caused by the surgery leading to decreased gastric capacity, decreased gastric emptying time (causing food to leave the stomach rapidly and possibly incompletely processed, into the duodenum) and absence of receptive relaxation as well as alterations in the contractile activity in the proximal stomach owing to excision of the fundus. Recently, the hormonal basis for weight reduction after LSG has been gaining importance. LSG significantly reduces the production of the orexigenic hormone, ghrelin in 90% of patients in a durable fashion, thus reducing the orexigenic behaviour and inducing early satiety in obese individuals. It is probably the result of resecting the gastric fundus where the majority of ghrelin production takes place [14–16].
Effect on Metabolic Profile:
LSG has been shown to reduce comorbidities and mortality in patients with morbid obesity and most significantly to ameliorate or resolve T2DM [17–19]. Much of the improvement has been related to the excess weight loss after surgery. However, some effects appear to be independent from weight loss.
In our study, there was significant reduction in levels of glycosylated haemoglobin at three months and six months postoperatively in diabetic patients with mean HbA1C at three and six months being 6.53% and 5.51% respectively as compared to the mean preoperative value of 7.98%.
Similar reduction in glycosylated haemoglobin levels was found in a study conducted by Schauer PR et al., who showed a rapid improvement in levels of glycosylated haemoglobin from 9.5% to 7.1% at three months and 6.7% at six months after LSG [20].
Shah S et al., Todkar JS et al., Basso N et al., and Berry M et al., also showed a decrease in glycosylated haemoglobin levels after LSG, thus signifying the role of LSG in improvement and remission of diabetes [21–24].
The achievement of normoglycaemia after bariatric procedures results from multiple changes that occur postoperatively such as dietary control, decreased plasma ghrelin levels, which in turn leads to increase in maximal capacity of glucose induced insulin release by the islet cells. Other causes of normoglycaemia include weight loss, reduction of body fat and the release of gastrointestinal hormones that interfere with the function of pancreatic beta-cells (incretins).
Our study also showed a significant improvement in hypertension and a decrease in systolic blood pressure. Our results are in accordance with the studies conducted by Srinivasa S et al., who reported a 29% resolution and 48% improvement in hypertension one year after LSG [25], Boza C et al., who reported a 62.5% resolution of arterial hypertension at one year after LSG [13] and D’Hondt M et al., who reported a 95% resolution/improvement in hypertension status one year after sleeve gastrectomy [26].
Dyslipidemia is a recognized cardiovascular risk factor in obese patients. In our study, there was a significant decrease in serum cholesterol, serum triglycerides, serum VLDL and serum LDL levels at six months with a significant improvement in serum HDL levels. Out of a total of 23 dyslipidemic patients, 20 patients (86.95%) achieved normal serum cholesterol levels (<240 mg/dl) within six months of surgery and thus showed a resolution of dyslipidemia. Similar results were found in a study conducted by Chowbey PK et al., who showed resolution of dyslipidemia in 34% of the patients with significant decrease in mean cholesterol and LDL levels six months after LSG [27]. Todkar JS et al., conducted a study on 20 dyslipidemic patients who underwent LSG. Significant decrease in serum cholesterol, triglycerides and LDL levels was observed with an increase in HDL levels [22]. Similarly, Hady H et al., also observed significant decrease in serum cholesterol, LDL and serum triglyceride levels with an increase in serum HDL levels following sleeve gastrectomy in 130 obese patients undergoing LSG [28].
Effect On Anthropometric Parameters:
In our study, mean waist circumference of 91 obese patients was 1.37±0.16 metres preoperatively. Postoperatively at one week, the mean waist circumference decreased to 1.31±0.15 metres but there was a significant decrease in waist circumference at three and six months following surgery with the mean values falling down to 1.12±0.13 and 0.99±0.08 respectively. There was a significant decrease in waist to hip ratio at three and six months postoperatively, from mean preoperative value of 1.07±0.06 to mean values of 0.98±0.04 and 0.94±0.04 at three and six months respectively. This signifies that LSG as a sole bariatric procedure decreases the risk of central obesity. In our study, there is no significant change in hip circumference of the patients. There is not enough data in literature to support our result but probably it is a means to calculate the waist-hip ratio which is an important indicator of central obesity.
Similar to our study, Miguel G et al., showed a significant decrease in mean waist circumference from 118.42±5.71 cm to 89.87±6.66 cm one year after the surgery [29]. However in our study, we saw a significant decrease as early as six months after surgery which was the stipulated time of our follow up.
Similar findings were documented in a clinical report published in 2012 by Hady H et al. After one year of the surgery, waist circumference in women decreased from 122.8±18.4 cm to 89±8.2 cm and in men from 134.2±27.6 cm to 106±9.66 cm [28].
Limitation
It was a short term follow up and may not reflect long term effects on weight loss and metabolic profile produced by LSG. Our sample size was relatively smaller. The findings need to be extrapolated on a larger cohort before these can be standardized and recommended. Lastly, the observational error and subjective bias cannot be overlooked as the study involved multiple laboratory and observer related measurements which could produce some discrepancy in results.
Conclusion
LSG is a good weight loss technique in obese and super obese patients as it produces sustainable weight loss with lesser complications both intraoperatively and postoperatively. As a sole bariatric procedure, LSG leads to significant improvement in glycaemic status and even resolution of diabetes in majority of population. LSG also brings about control of dyslipidemia and metabolic syndrome in severe to morbidly obese patients. LSG is efficacious in reducing central obesity in Indian population which is a major depressive ailment amongst obese individuals.