Role of CD10 Immunoexpression in Grading Phyllodes Tumour of the Breast
Maithili Mandar Kulkarni1, Siddhi Gaurish Sinai Khandeparkar2, Avinash R Joshi3, Vishakha Kothikar4, Anuja Nasare5, Sukhada Patil6, Supriya Niraspatil7, Bhagyashree Dhande8
1 Asssociate Professor, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
2 Asssociate Professor, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
3 Professor, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
4 Postgraduate Student, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
5 Postgraduate Student, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
6 Postgraduate Student, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
7 Postgraduate Student, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
8 Postgraduate Student, Department of Pathology, Smt Kashibai Navale Medical College and General Hospital, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Maithili Mandar Kulkarni, E-401, Samrajya, Shivtirthnagar, Paud Road, Kothrud-411038, Pune, Maharashtra, India.
E-mail: drmaithili@rediffmail.com
Introduction
Fibroepithelial tumours are a heterogeneous group of biphasic neoplasms consisting of a proliferation of both epithelial and stromal components. Fibroadenoma (FA) and Phyllodes Tumour (PT) constitute the major entities. It is crucial to distinguish benign from borderline PT (low grade malignant PT), because the former do not metastasize, have a lesser risk of local recurrence and initial local recurrences are histologically benign in almost all instances. Multiple Immunohistochemical (IHC) markers are being studied to find their utility in grading the PT accurately for planning proper treatment.
Aim
To study, the IHC expression of CD10 in the stromal cells of a series of PTs and FA, with the aim of determining whether the degree of CD10 expression in the stromal cells is related to the grade of the tumour.
Materials and Methods
Records of 28 cases of PT and 35 cases of FA received in the Department of Pathology in a tertiary care hospital were obtained. Histopathology reports and slides of all the cases were reviewed and clinical data such as age and histomorphological features such as tumour cellularity, stromal overgrowth, mitotic count and nuclear atypia were noted. Representative block of the tumour with maximum cellularity was subjected to CD10 staining. For FA and benign PT a technique of tissue microarray was used. For borderline and malignant PT, representative section was used. Stromal cell staining was assessed, using cytoplasmic staining of the breast myoepithelium as internal control.
Results
Present study included 35 cases of FA, 20 cases of benign PT, five cases of borderline PT and three cases of malignant PT. The mean age of the patients increased with the increasing tumour grade of PT and this was also observed for FA and benign PT. The mean age increased with increase in tumour grade of PT and was statistically significant (p<0.05). The mean size did not increase with the increasing tumour grade of PT and was statistically insignificant (p=0.0429). Mean tumour size was more in benign PT as compared to FA and was highly statistically significant (p<0.01). CD10 staining was diffuse (Grade-3) and strong in malignant PT. The staining intensity was strong but patchy (Grade-2) in borderline PT. Weak and patchy (Grade-1) CD10 staining was seen in four benign PT and six FA. Other cases of benign PT and FA were negative for CD10 immunoreactivity.
Conclusion
Our study showed that CD10 expression strongly correlates with the PT grade, which can help in the differentiation between benign and malignant variants of PT.
Benign, Borderline, Fibroadenoma, Malignant
Introduction
Fibroepithelial tumours are a heterogeneous group of biphasic neoplasms consisting of a proliferation of both epithelial and stromal components [1]. FA and PT constitute the major entities. FA is a common benign biphasic tumour. PT are classified into benign, borderline and malignant categories on the basis of a combination of histological features, including the degree of stromal hypercellularity, mitosis and cytological atypia, stromal overgrowth and nature of the tumour borders/margins [1]. Borderline and frankly malignant PT can metastasize, the incidence being 4% and 22% respectively [2,3]. Histological criteria used for grading PT lack standard interpretation and there is an inter-observer variability among pathologists [4]. Multiple IHC markers are being studied to find their utility in grading the PT accurately for planning proper treatment [5–9]. The fundamental principle of therapy is complete excision with safe margins to prevent local recurrence [2]. Malignant PT may require frequent follow up visits and additional radiotherapy and/or chemotherapy [10].
The utility of various IHC markers to distinguish benign from malignant PT has been studied. CD10 is zinc-dependent peptidase (metalloproteinase), which degrades a variety of bioactive peptides. CD10 has been evaluated and increased expression has been observed with increasing tumour grade of PT. Few such studies have been documented in literature so far which need validation [5,7,9–12].
In present study, the IHC expression of CD10 was evaluated in the stromal cells of a series of PTs and FAs, with the aim of determining whether the degree of CD10 expression in the stromal cells is related to the grade of tumour.
Materials and Methods
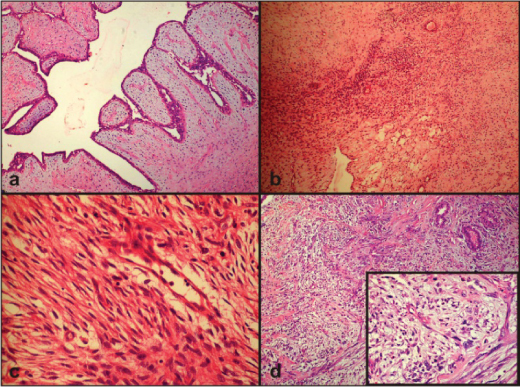
This is a cross-sectional study. Records of 28 cases of PT and 35 cases of FA received over a period of five years (2012-2016) in the Department of Pathology in a Smt. Kashibai Navale Medical college and General Hospital, Pune, Maharashtra, India, were obtained. Histopathology reports and slides of all the cases were reviewed and clinical data such as age and histomorphological features such as tumour cellularity, stromal overgrowth, mitotic count and nuclear atypia were noted. PT cases were divided into three categories including benign [Table/Fig-1a], borderline [Table/Fig-1b,c] and malignant [Table/Fig-1d] as described previously [2]. Representative block of the tumour with maximum cellularity was subjected to CD10 staining. For FA and benign PT a technique of tissue microarray was used [13]. For borderline and malignant PT representative section was used. Stromal cell staining was assessed, using cytoplasmic staining of the breast myoepithelium as internal control. The staining intensity was graded as 0 (no staining), weak, moderate or strong if the staining was much weaker, slightly weaker, or of the same intensity as that of the myoepithelium respectively. The percentage of stained cells was also assessed as negative (when there was no stromal staining), Grade-1 (<10% stromal cells were positive), Grade-2 (10 to 30% stromal cells were positive), and Grade-3 (>30% stromal cells were positive) [9].
Photomicrograph showing: a) Benign PT with no stromal overgrowth and no mitosis (H&E, 100x); b) Photomicrograph showing borderline PT (H&E, 100x); c) Photomicrograph showing borderline PT (H&E, 400x); d) Photomicrograph showing malignant PT with marked stromal overgrowth, nuclear atypia, mitosis (H&E, 100x), inset (H&E, 400x).
Statistical Analysis
The statistical software named Primer software version 5.0 (manufactured by McGraw-Hill) was used for analyzing the data. The groups were compared using the Pearson’s chi-square test (PCT) or Fisher’s-Exact-test (FET). ANOVA test was used to compare the difference in mean between multiple groups and t-test was used to compare the difference in mean between two groups. A p-value of 0.05 or less was considered statistically significant.
Results
Present study included 35 cases of FA, 20 cases of benign PT, five cases of borderline PTs and three cases of malignant PT. Age range of FA was 18 to 45 years with mean age of 25 years. Mean tumour size of FA was 2.92±1.26 cm. Relation between patients age and tumour size with tumour grade of PT is compared in [Table/Fig-2,3] respectively.
Relation between patients age and tumour grade of PT.
| Tumour type | Youngest age (years) | Oldest age (years) | Mean±SD |
|---|
| Benign PT | 18 | 56 | 34±11.25 |
| Borderline PT | 40 | 50 | 45±5 |
| Malignant PT | 45 | 55 | 49.67±5.03 |
F=4.76, p<0.05 (ANOVA)
Relation between tumour size and tumour grade of PT.
| Tumour type | Smallest size (cm) | Largest size (cm) | Mean±SD |
|---|
| Benign PT | 2 | 13 | 5.2±3.17 |
| Borderline PT | 3 | 11 | 7.2±3.34 |
| Malignant PT | 4 | 10 | 6.17±2.36 |
F=0.87, p=0.429 (ANOVA)
Relation between CD10 expression in PT and FA and Ki67 labeling index in PT with tumour grade is given in [Table/Fig-4,5] respectively.
Relation between CD10 expression and tumour grade of PT and FA.
| Tumourtype | CD10positive (%) | CD10negative (%) | Total(%) | Statistical analysis |
|---|
| Benign PT | 4(20%) | 16(80%) | 20(100) | χ2=14.933,p<0.001 |
| Borderline PT | 5(100%) | 0 | 5(100) |
| Malignant PT | 3(100%) | 0 | 3(100) |
| Fibroadenoma | 6(17%) | 29 (83%) | 35(100) | χ2=0.0698,p=0.7915 |
Chi-square test
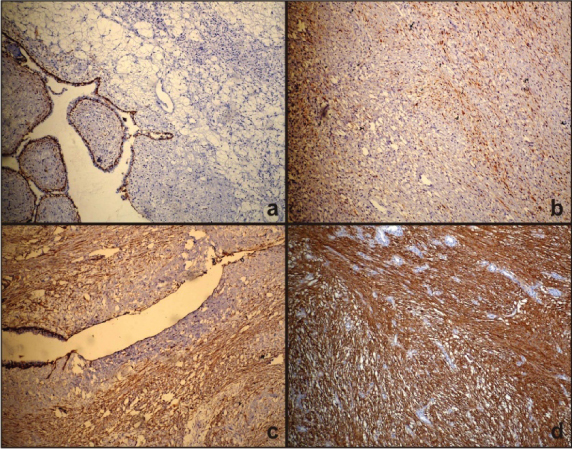
Photomicrograph showing: a) Negative CD10 staining (100x); b) Photomicrograph showing weak and patchy CD10 (Grade-1) staining in benign PT (100x); c) Photomicrograph showing strong and patchy CD10 (Grade-2) staining in borderline PT (100x); d) Photomicrograph showing strong and diffuse CD10 (Grade-3) staining in malignant PT (100x).
The mean age increased with increase in tumour grade of PT and was statistically significant (p<0.05). The mean size did not increase with the increasing tumour grade of PT and was statistically insignificant (p=0.0429). Mean tumour size was more in benign PT as compared to FA and was highly statistically significant (p<0.01). Mean age of patients with benign PT was more than that in FA and this was highly statistically significant (p<0.01).
Few cases of benign PT and FA were negative for CD10 immunoreactivity [Table/Fig-5a], Weak and patchy (Grade1) CD10 staining was seen in four benign PT and six FA [Table/Fig-5b]. The staining intensity was strong but patchy (Grade-2) in borderline PT [Table/Fig-5c], CD10 staining was diffuse (Grade-3) and strong in malignant PT [Table/Fig-5d].
Discussion
The median age and mean age of PTs are known to be about 45 years, approximately 20 years older than the median age of patients with FA [2]. Relatively younger age group is observed in Asian and Latin patients [2]. In our study, the age correlated with the tumour grade and was statistically significant. Average size of PT is reported as 4 to 5 cm with a range of 1 to 20 cm in literature [2]. Although malignant PTs tend to be larger than benign variants, there are many exceptions with high grade malignant lesions smaller than 2 cm and largest lesions histopathologically benign [2]. In present study, mean size did not increase with increase in tumour grade and was not statistically significant. Similar observation was found in study done on 67 cases of PT [11].
Earlier studies suggested that CD10 expression in tumour stroma was associated with aggressive biological behaviour [9]. CD10 expression seen in the stromal cells of invasive breast carcinoma was associated with an increased incidence of lymph node metastasis [7]. The CD10 expression in the stromal cells of fibroepithelial lesions of the breast has been reported in very few studies. [5,7,9–12].
In one large study, it was observed that CD10 expression was low in both FA and benign PT and was much higher in borderline and malignant PT [7]. This was in correlation with clinical evidence that both borderline and frankly malignant PT can metastasize, whereas, benign PT and FA do not metastasize [7]. We found similar observations in our study. CD10 expression does not assist in the differential diagnosis between FA and PT. Because of the limited data available, the percentage of benign versus malignant PT was not well defined. Reports suggest that about 85-90% of PT are benign and about 10-15% are malignant [14]. Our study shows that CD10 expression strongly correlates with the PT grade, which can help in the differentiation between benign and malignant variants. This is evidenced by the fact that CD10 immunoreactivity was seen in 20% of the benign PT, 100% of the borderline PT and 100% of malignant PT. This may be attributed to the fact that CD10 belongs to the metalloprotease family, so it provides tumours with the capacity to invade blood vessel walls, facilitating its metastatic potential [7].
There was significant increase in CD10 expression as the lesions progressed from benign PT to borderline and frank malignancy. All the malignant cases showed strong and diffuse intensity for CD10. All borderline cases showed patchy but strong immunoreactivity. Benign cases showed patchy and weak immunoreactivity. Similar observations were found in study done on 25 cases of PT [12].
In breast, CD10 is often adopted as a marker for myoepthelial cells in differential diagnosis. Not Otherwise Specified (NOS) type of sarcoma with CD10 expression (NSCD10) is a variant of sarcoma with myoepithelial differentiation. The expression of CD10 in NSCD10 is known to be very extensive and intensive than those of malignant PTs. CD34 is valuable marker to differentiate between the two as PTs are CD34 immunoreactive. Nevertheless meticulous histopathological evaluation for the presence of benign glandular elements helps confirm the diagnosis of malignant PT [15]. Sarcomatoid metaplastic carcinomas have spindled component which is positive for high molecular weight keratin or p63 and thus can be differentiated from malignant PT [1].
Limitation
The limitation of the present study is the small sample size of the borderline and malignant PT owing to their rarity.
Conclusion
Our study shows that stromal CD10 expression pattern and intensity strongly correlates with the PT grade, which can help in the differentiation between benign and malignant variants of PT. Lesions with ability to metastasise (borderline and malignant PTs) show much greater CD10 expression than those without this ability (benign PT and FA). Thus, CD10 immunoreactivity can assist the meticulous histopathological examination to accurately grade the PTs and help in planning careful follow up and adequate treatment.
F=4.76, p<0.05 (ANOVA)
F=0.87, p=0.429 (ANOVA)
Chi-square test
[1]. Fattaneh A, Devilee P, WHO classification of tumours: Tumours of the breast and female genital organs 2003 LyonIARC [Google Scholar]
[2]. Rosen PP, Secretory carcinomaIn: Rosen’s Breast Pathology 2009 3rd edPhiladelphia, PALippincott Williams and Wilkins:563-70. [Google Scholar]
[3]. Moffat CJ, Pinders SE, Dixon AR, Elston CW, Blamey RW, Ellis IO, Phyllodes tumours of the breast: A clinico-pathological review of thirty-two casesHistopathology 1995 27(3):205-18. [Google Scholar]
[4]. Ortega E, Aranda FI, Chuliá MT, Niveiro M, Payá A, Seguí J, Phyllodes tumour of the breast with actin inclusions in stromal cells: Diagnosis by fine needle aspirationDiag Cytopathol 2001 25(2):115-17. [Google Scholar]
[5]. Al-Masri M, Darwazeh G, Sawalhi S, Mughrabi A, Sughayer M, Al-Shatti M, Phyllodes tumour of the breast: Role of CD10 in predicting metastasisAnn Surg Oncol 2012 19(4):1181-84. [Google Scholar]
[6]. Chen CM, Chen CJ, Chang CL, Shyu JS, Hsieh HF, Harn HJ, CD34, CD117, and actin expression in phyllodes tumour of the breastJ Surg Res 2000 94(2):84-91. [Google Scholar]
[7]. Tse GM, Tsang AK, Putti TC, Scolyer RA, Lui PC, Law BK, Stromal CD10 expression in mammary fibroadenomas and phyllodes tumoursJ Clin Pathol 2005 58(2):185-89. [Google Scholar]
[8]. Millar EK, Beretov J, Marr P, Sarris M, Clarke RA, Kearsley JH, Malignant phyllodes tumours of the breast display increased stromal p53 protein expressionHistopathology 1999 34(6):491-96. [Google Scholar]
[9]. Ibrahim WS, Comparison of stromal CD10 expression in benign, borderline, and malignant phyllodes tumours among Egyptian female patientsIndian J Pathol Microbiol 2011 54(4):741-44. [Google Scholar]
[10]. Tariq MU, Haroon S, Kayani N, Role of CD10 immunohistochemical expression in predicting aggressive behaviour of phylloides tumoursAsian Pac J Cancer Prev 2015 16(8):3147-52. [Google Scholar]
[11]. Hussin H, Pailoor J, Cheng PS, The role of CD10 immunohistochemistry in the grading of phyllodes tumour of the breastJ Interdiscipl Histopathol 2013 1(4):195-203. [Google Scholar]
[12]. Puri V, Jain M, Mahajan G, Pujani M, Critical appraisal of stromal CD10 staining in fibroepithelial lesions of breast with a special emphasis on expression patterns and correlation with WHO gradingJ Can Res Ther 2016 12(2):667-70. [Google Scholar]
[13]. Pathak GS, Deshmukh SD, Ashturkar AV, Construction of tissue arrays without prefabricated recipient paraffin block experience of a novel technique in resource poor settingsIndian J Pathol Microbiol 2011 54(3):654-55. [Google Scholar]
[14]. Liang MI, Ramaswamy B, Patterson CC, McKelvey MT, Gordillo G, Nuovo GJ, Giant breast tumours: Surgical management of phyllodes tumours, potential for reconstructive surgery and a review of literatureWorld J Surg Oncol 2008 6:117 [Google Scholar]
[15]. Yang GZ, Li J, Jin H, Ding HY, Is mammary not otherwise specified-type sarcoma with CD10 expression a distinct entity? A rare case report with immunohistochemical and ultrastructural studyDiagn Pathol 2013 8:14 [Google Scholar]