Aerococcus Viridans: A Rare Pathogen Causing Urinary Tract Infection
Balvinder Mohan1, Kamran Zaman2, Naveen Anand3, Neelam Taneja4
1 Associate Professor, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
2 Senior Resident, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
3 Junior Resident, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
4 Professor, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Balvinder Mohan, Associate Professor, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh-160012, India.
E-mail: balvindermohan2002@yahoo.com
Aerococci are Gram-positive cocci with colony morphology similar to viridans streptococci. Most often these isolates in clinical samples are misidentified and considered insignificant. However, with the use newer techniques like Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass-Spectrometry (MALDI-TOF MS), aerococci have been recognized as significant human pathogens capable of causing a diverse spectrum of infections. Among the different species of aerococci, Aerococcus urinae is the most common agent causing Urinary Tract Infection (UTI) followed by A. sanguinocola. Aerococcus viridans (A. viridans) have been reported rarely in urinary tract infections. The antimicrobial resistance in aerococci in terms of its intrinsic resistance and evolving resistance to penicillin and vancomycin has raised the concern for better understanding of this pathogen. We recently encountered two cases of nosocomial UTI caused by A. viridans which are being reported here.
Aerococci, Nosocomial, Vancomycin
UTI form a major component of the most commonly encountered bacterial infections in routine clinical practice. Most of these infections are caused by members of the Enterobacteriaceae family; in particular, Escherichia coli and the Gram-positive cocci such as Staphylococcus spp and Enterococcus spp [1]. In routine clinical laboratories, the Gram-positive, catalase negative urinary isolates with pinhead size colonies and α-haemolytic features are usually identified as Streptococcus spp or Enterococcus spp. Recently, the use of MALDI-TOF MS has led to the identification of some of these isolates as aerococci [2,3]. In the past, exact identification of these organisms was difficult and most of the isolates were not identified correctly, therefore, their clinical significance remained obscure. However, aerococci are now being increasingly recognized as human pathogens with the use of MALDI-TOF [3,4]. They are reported to cause various invasive infections like infective endocarditis, bacteraemia, urosepsis in elderly and rarely osteomyelitis, joint infections, skin-soft tissue infections and others [2–9]. Though isolated rarely from urine cultures, the clinical significance of aerococci as uropathogen has been doubtful. Of the different species of aerococci, Aerococcus urinae is the most common agent causing UTI followed by A. sanguinocola. A. viridans has been reported rarely in UTI [2,10,11]. In India, till date in literature, A. viridans has been reported in a case of infective endocarditis [6]. We are the first to report the cases of A. viridans causing nosocomial UTI from India.
The present case reports have been retrospectively reviewed and reported and it did not require any institutional ethics committee approval. The patients involved in this report have given their written informed consent authorizing use and disclosure of their protected health information.
Case-1
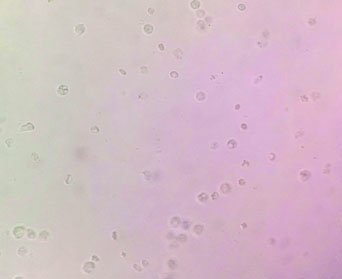
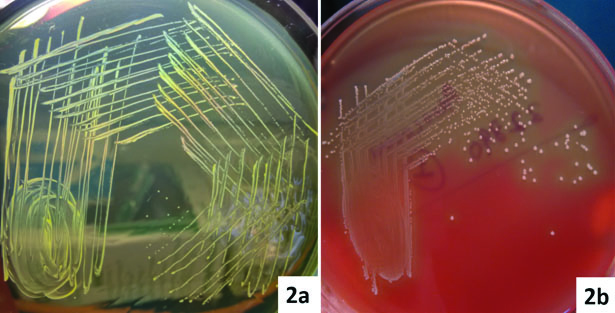
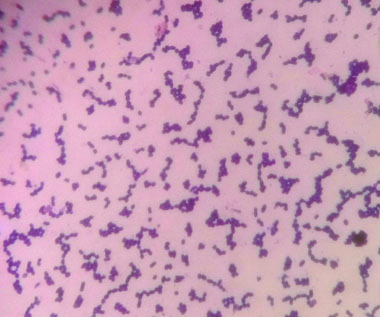
A 48-year-old female was admitted to the surgical ward for intestinal obstruction. She underwent exploratory laparotomy in which gangrenous ileal loop was resected. A subtotal colectomy and end ileostomy with distal mucosal fistula was performed. On the fourth postoperative day, she complained of increased frequency of micturition and fever. There was no evidence of superficial surgical site infection. Clinical suspicion of intra-abdominal infection was made and the workup for the postoperative fever was carried on the same day which showed Total Leucocyte Count (TLC) of 13200 cells/mm3. The patient was started empirically on parental piperacillin-tazobactam (3.375g IV q.i.d) and amikacin (15 mg/kg IV b.d). The blood sample for culture was collected prior to the start of therapy. The blood culture was carried out in an automated blood culture system, the blood culture was found to be sterile even after five days of incubation. The urine sample subjected to microbiological testing, on wet mount microscopic examination numerous pus cells and bacteria per high-power field were observed [Table/Fig-1]. The semi-quantitative culture done on the Cysteine Lysine Electrolyte Deficient (CLED) agar showed significant bacterial growth (colony count >105 CFU/ml). The colonies were small (1-2 mm) yellowish in colour [Table/Fig-2a]. The isolate was subcultured on 5% sheep blood agar, which showed 1-2 mm size, circular, α-haemolytic colonies [Table/Fig-2b]. On Gram’s stain, they were Gram-positive cocci of approximate 1 μm in size arranged in clusters [Table/Fig-3], catalase negative and were bile esculin negative. The isolate was subjected to identification by MALDI-TOF MS instrument (Bruker Daltonics, Bremen, Germany). The isolate was confirmed as A. viridans with identification score of ≥ 2.2. A set of antibiotics were chosen as there are no available CLSI guidelines and antimicrobial susceptibility was carried out by Kirby-Bauer disc diffusion method. The isolate was sensitive to amoxicillin, vancomycin, tetracycline and carbapenems and resistant to nitrofurantoin, ciprofloxacin, cotrimoxazole, clindamycin, erythromycin, and gentamycin. The patient was started on intravenous amoxicillin (500 mg t.i.d) and responded well. On eighth postoperative day, the patient was discharged on oral amoxicillin (500 mg t.i.d) for five days. The follow-up urine culture after a week was sterile.
Wet mount of urine samples showing numerous pus cells in both the cases.
a) Colonies of A. viridans from the urine samples on CLED medium; b) On 5% sheep blood agar showing 1-2 mm size, α-haemolytic colonies of A. viridans.
Gram’s stain of A. viridans colonies showing Gram-positive cocci of approximate 1 μm in size arranged in clusters.
Case-2
A 49-year-old male who had sustained head injury with subdural haemorrhage after road traffic accident underwent cranial surgery. A Foley’s urinary catheter was placed before the surgery. On the fourth postoperative day, the patient had high-grade fever with clinical deterioration. He was screened for sepsis, which revealed raised TLC (14,100 cells/mm3) and blood culture was sterile. On fifth postoperative day, the catheter was changed over to a condom catheter and the urine sample collected was sent for culture sensitivity to rule out UTI. The wet mount microscopic examination showed few red blood cells, numerous pus cells and bacteria per high-power field. The semi-quantitative culture done on CLED media showed significant bacterial growth (>105 CFU/ml). The colony morphology on CLED/ blood agar and other feature of this isolate were similar to the isolate in the first case. This isolate was confirmed as A. viridans (identification score of 2.3) using MALDI-TOF. Antimicrobial susceptibility was carried out as described earlier in the first case. The isolate was sensitive to vancomycin, tetracycline, and carbapenems and resistant to nitrofurantoin, amoxicillin, ciprofloxacin, cotrimoxazole, clindamycin, erythromycin and gentamycin. The patient was started on vancomycin (500 mg every six hours). The patient responded well to treatment and was discharged on the 10th postoperative day.
Discussion
In our present case series, both patients were young and had developed nosocomial UTI after four days of surgery. Both the cases were from the same ward in the hospital. Aerococci have been shown to be widely distributed in the hospital environment and can lead to infections like endocarditis, urinary tract infection and bacteraemia [12]. However, the infection in both cases was localized to the urinary tract with no features of bacteraemia, as the blood cultures were sterile. The association of aerococcal bacteriuria with bacteraemia is less often noted except in a case of elderly females [13,14].
As there are no CLSI guidelines available till now for Aerococci and antimicrobial susceptibility was carried out by Kirby-Bauer disc diffusion method for a set of antibiotics as per the available literature [10,13]. The isolate in the first case was sensitive to amoxicillin, vancomycin, tetracycline and carbapenems and the second case isolate was sensitive to vancomycin, tetracycline and carbapenems only. Most of the aerococcus isolates are found to be susceptible for penicillins and carbapenems, however, they show higher MICs for cephalosporins. Aerococci are known to have high MICs or resistance to cotrimoxazole, ciprofloxacin, tetracycline, clindamycin and gentamycin [15–20]. Resistance to penicillin and vancomycin has been reported earlier [16–19]. Interesting to note was both of our isolates were resistant to nitrofurantoin which is considered to be first line drug in patients with uncomplicated UTI [2] and both the isolates in our case series were sensitive to tetracycline. Earlier misidentification of aerococci may have led not only to under-reporting but also doubtful clinical significance as uropathogen. This study highlights the role of MALDI-TOF for identifying unusual organisms and a better clinical association as shown in the previous studies [3,4]. In patients where aerococci are isolated in significant numbers from urine, with fever and no other foci of infection are detected, especially in a hospital setting then antimicrobial therapy should be given in accordance with the susceptibility results in view of emerging resistance to drugs. This study also emphasizes on the fact that the CLSI/EUCAST susceptibility guidelines need to be formulated taking into consideration these rarely isolated microorganisms.
Conclusion
Aerococci are often misidentified and considered insignificant due to similarity with viridans streptococci. MALDI-TOF MS have now recognized aerococci as significant human pathogens capable of causing a wide range of infections. Knowledge about the newer microbial agents, their epidemiology and susceptibility profiles, will supplement in formulating appropriate antibiotic treatment regimens and policies.
[1]. Warren JW, Clinical presentations and epidemiology of urinary tract infection. In: Mobley HL, Warren JW, editorsUrinary tract infections molecular pathogenesis and clinical management 1996 Washington DCAmerican Society for Microbiology Press:3-27. [Google Scholar]
[2]. Rasmussen M, Aerococcus: An increasingly acknowledged human pathogenClin Microbiol Infect 2016 22:22-27. [Google Scholar]
[3]. Senneby E, Nilson B, Petersson AC, Rasmussen M, Matrix-assisted laser desorption ionization-time of flight mass spectrometry is a sensitive and specific method for identification of aerococciJ Clin Microbiol 2013 51(4):1303-04. [Google Scholar]
[4]. Senneby E, Göransson L, Weiber S, Rasmussen M, A population-based study of aerococcal bacteraemia in the MALDI-TOF MS-eraEur J Clin Microbiol Infect Dis 2016 35(5):755-62. [Google Scholar]
[5]. Untereker WJ, Hanna BA, Endocarditis and osteomyelitis caused by Aerococcus viridansMt Sinai J Med 1976 43:248-52. [Google Scholar]
[6]. Augustine T, Thirunavukkarasu B, Bhat Vishnu, Bhatia B.D, Aerococcus viridans endocarditisIndian pediatrics 1994 31:599-601. [Google Scholar]
[7]. Santos R, Santos E, Gonçalves S, Marques A, Sequeira J, Abecasis P, Lymphadenitis caused by Aerococcus urinae infectionScand J Infect Dis 2003 35:353-54. [Google Scholar]
[8]. Schuur PM, Sabbe L, van der Wouw AJ, Montagne GJ, Buiting AG, Three cases of serious infection caused by Aerococcus urinaeEur J Clin Microbiol Infect Dis 1999 18:368-71. [Google Scholar]
[9]. Jost C, Breton B, Biran V, Khung S, Chekroune S, Bonacorsi S, First case of pregnant women bacteraemia and probable early-onset neonatal infection due to Aerococcus urinaeNew Microbes New Infect 2015 3:1-3. [Google Scholar]
[10]. Senneby E, Petersson AC, Rasmussen M, Epidemiology and antibiotic susceptibility of aerococci in urinary culturesDiagn Microbiol Infect Dis 2015 81:149-51. [Google Scholar]
[11]. Gopalachar A, Akins RL, Davis WR, Siddiqui AA, Urinary tract infection caused by Aerococcus viridans, a case reportMedical Science Monitor: International Medical Journal of Experimental and Clinical Research 2004 10(11):CS73-75. [Google Scholar]
[12]. Kerbaugh MA, Evans JB, Aerococcus viridans in the hospital environmentAppl Microbiol 1968 16(3):519-23. [Google Scholar]
[13]. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG, Urinary tract infections with Aerococcus urinae in the South of the NetherlandsEur J Clin Microbiol Infect Dis 1997 16:871-75. [Google Scholar]
[14]. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL, Clinical significance of Aerococcus urinae: A retrospective reviewDiagn Microbiol Infect Dis 2005 53:289-92. [Google Scholar]
[15]. Shelton-Dodge K, Vetter EA, Kohner PC, Nyre LM, Patel R, Clinical significance and antimicrobial susceptibilities of Aerococcus sanguinocola and Aerococcus urinaeDiagn Microbiol Infect Dis 2011 70:448-51. [Google Scholar]
[16]. Humphries RM, Hindler JA, In vitro antimicrobial susceptibility of Aerococcus urinaeJ Clin Microbiol 2014 52:2177-80. [Google Scholar]
[17]. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F, In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamycin and vancomycinJ Antimicrob Chemother 2001 48:653-58. [Google Scholar]
[18]. Christensen JJ, Korner B, Casals JB, Pringler N, Aerococcus-like organisms: Use of antibiograms for diagnostic and taxonomic purposesJ Antimicrob Chemother 1996 38:253-58. [Google Scholar]
[19]. Lupo A, Guilarte YN, Droz S, Hirzel C, Furrer H, Endimiani A, In vitro activity of clinically implemented β-lactams against Aerococcus urinae: Presence of non-susceptible isolates in SwitzerlandNew Microbiol 2014 37:563-66. [Google Scholar]
[20]. Swanson H, Cutts E, Lepow M, Penicillin-resistant Aerococcus viridans bacteraemia in a child receiving prophylaxis for sickle-cell diseaseClin Infect Dis 1996 22:387-88. [Google Scholar]