Efficacy of Natural and Allopathic Antimicrobial Agents Incorporated onto Guided Tissue Regeneration Membrane Against Periodontal Pathogens: An in vitro Study
Neha Mehrotra1, Ajay Reddy Palle2, Rajani Kumar Gedela3, Sanjay Vasudevan4
1 Postgraduate Student, Department of Periodontics, Army College of Dental Sciences, Secunderabad, Telangana, India.
2 Reader, Department of Periodontics, Army College of Dental Sciences, Secunderabad, Telangana, India.
3 Reader, Department of Periodontics, Army College of Dental Sciences, Secunderabad, Telangana, India.
4 Professor and Head, Department of Periodontics, Army College of Dental Sciences, Secunderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Neha Mehrotra, Room No. 9, Lepakshi Girls Hostel, Army College of Dental Sciences, Jai Jawahar Nagar, Chennapur-Crpf Road, Secunderabad-500087, Telangana, India.
E-mail: drnehamehrotra18@gmail.com
Introduction
Periodontal disease is one of the most prevalent afflictions worldwide. It is an infection of the periodontium as a result of subgingival colonization of the specific microbiota, leading to loss of attachment, which requires optimal care for regeneration to its pre-disease state. Guided Tissue Regeneration (GTR) is one of the successful treatment modalities in Periodontal Regenerative Therapy, but is vulnerable to bacterial colonization. The conflict between usage of classical antibiotics and plant origin antimicrobial agents has recently been in the limelight.
Aim
The aim of this study was to assess the in vitro antimicrobial activity of amoxicillin, metronidazole and green coffee extract loaded onto GTR membrane against periodonto-pathogens.
Materials and Methods
Pure form of amoxicillin, metronidazole and green coffee extract were obtained. One percent concentration of each antimicrobial agent was prepared by appropriate dilution with distilled water. GTR membrane was cut into a size of 1x0.5 cm under sterile conditions and was coated with the antimicrobial agents respectively and with distilled water as the negative control. Antimicrobial activity was checked against Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and Porphyromonas gingivalis (P. gingivalis) using agar disc diffusion method. The statistical analysis was done using Kruskal Wallis ANOVA and Mann-Whitney U test.
Results
One percent amoxicillin showed level of significance (p>0.05) against both A. actinomycetemcomitans and P. gingivalis. Green coffee extract showed no zone of inhibition against both the bacterial species.
Conclusion
Loading of commercially available antimicrobial agents onto GTR membrane can prevent its bacterial colonization leading to better treatment outcomes for periodontal regeneration.
Amoxicillin, Aggregatibacter actinomycetemcomitans, Green Coffee extract, Metronidazole, Porphyromonas gingivalis
Introduction
It has been well established that oral infections, particularly periodontitis, are associated with aerobic and anaerobic microbiota. The oral cavity harbors more than 700 different types of bacterial species that are associated with various oral diseases [1]. The major putative periodonto-pathogens are A. actinomycetemcomitans and P gingivalis. A. actinomycetemcomitans is particularly associated with an inflammatory disease, localized aggressive periodontitis, which can result in early tooth loss [2]. P. gingivalis is one of the culprit organisms in chronic periodontitis and peri- implantitis [3,4]. An immune response is triggered by fibroblasts and macrophages as soon as the microbiota inhabits the periodontal tissues resulting in production of mediators of inflammation and immune response i.e., Interleukin (IL) 1, 6 and Tumour Necrosis Factor-α (TNF-α) [5].
GTR has been shown to be a successful treatment modality for periodontal regeneration in many clinical studies. The GTR technique prevents the apical migration of the gingival epithelium and allows the periodontal cells to repopulate in the area of the denuded root surface. Some of the human biopsy GTR studies have demonstrated new attachment level with bone fill [6,7]. The most common periodontal pathogens to inoculate on the GTR membrane are A. actinomycetemcomitans and P gingivalis [8]. A clinical study [9], reported that within three minutes of the procedure there is bacterial contamination of the GTR membrane. Hence, incorporating antimicrobial agents in the GTR membrane may help in preventing the surface colonization.
Several studies [10–12] have been reported that there is drastic reduction in colonization of periodontal pathogens on the GTR membrane and improvement in clinical sites followed by allopathic antimicrobial coating. The main concept that has instigated the search of new antimicrobial alternatives of plant origin is the emanation of drug resistance in pathogenic bacteria and the undesirable side effects of synthetic antimicrobial agents [13].
GTR membrane incorporated with antimicrobial agents may be beneficial to control membrane-associated infections in GTR therapy. The aim of this in vitro study was to determine whether the growth of A. actinomycetemcomitans and P. gingivalis can be prevented by the surface treatment of GTR membrane with antimicrobial agents.
Materials And Methods
An in vitro experimental design was formulated for the study. Institutional Ethics Committee of Army College of Dental Sciences, Secunderabad, Telangana, India, granted ethical clearance for conducting the study.
Pure form of amoxicillin and metronidazole was procured for the purpose of the study. Pure green coffee extract was procured from Top secret nutrition supplements, New York, USA containing 50% Chlorogenic Acid (CGA). The manufacturer certified it to be free from any form of bacteria, yeast or mold after microbial analysis. To obtain 10% concentration of antimicrobial agent, 1 gram of these antimicrobial agents was dissolved in 10 ml of distilled water. Further, appropriate amount of solvent was added to dilute the concentrations to 1% amoxicillin, 1% metronidazole and 1% green coffee extract respectively. Microbial assay was done of the extracts. Distilled water, the solvent was used as negative control in this study.
GTR membrane that was used for the study was sterile reconstituted Type-I collagen membrane. The membrane was aseptically cut into the dimensions of 1x0.5 cm. Nearly, 10 ml of 1% amoxicillin, 1% metronidazole and 1% green coffee extract solution was poured into sterile petridishes respectively in which the GTR membrane was placed. The GTR membrane was allowed to soak in the concentrated solution for 15 minutes and was dried for five minutes.
Microbial Assay
Agar disc diffusion method was used to determine the antimicrobial activity of GTR membrane coated with 1% amoxicillin solution, 1% metronidazole solution and 1% green coffee extract solution respectively in vitro. Blood agar was used for culturing and examining different microorganisms. Colonies of microorganisms were transferred to the agar plate by means of an inoculation loop. For ensuring even distribution of microorganisms the plates were rotated at approximately 60° between every streak. For A. actinomycetemcomitans and P. gingivalis, similar overall procedure of inoculum preparation and inoculation of culture media was followed. Both the bacteria were inoculated on three different agar plates of 1% amoxicillin solution, 1% metronidazole solution and 1% green coffee extract solution respectively. Therefore, for both the bacteria a total of six plates were inoculated. The inoculated plates were allowed to stand for at least three minutes but no longer than 15 minutes, before placing the GTR membrane treated with different antimicrobial substances to be tested. Within 15 minutes of the placement of GTR membrane, the plates were incubated at 37°C. For aerobic (A. actinomycetemcomitans) and anaerobic (P. gingivalis) bacteria, incubation was done for 48 hours. McIntosh and Filde’s anaerobic jar was used for anaerobic microorganism culturing while incubator was used at 37°C for aerobic microorganism for 48 hours. If the lawn of growth was confluent after the incubation period the plates were read. The diameter of zone of inhibition was measured to the nearest millimeter using Vernier’s caliper. The microbiological procedure was repeated three times for each bacterium. The control for the study was, GTR membrane treated with distilled water.
Statistical Analysis
For statistical analysis of the study, software Statistical Package for Social Sciences (SPSS 20.0 version) was used. The statistical data was obtained and analyzed using Kruskal Wallis ANOVA and Mann-Whitney U test. Mann-Whitney U test was done to compare the antimicrobial property between various groups. Statistical significance level was established at p<0.05.
Results
Zones of inhibition for 1% amoxicillin, 1% metronidazole, 1% green coffee extract and distilled water against A. actinomycetemcomitans and P. gingivalis are elaborated in [Table/Fig-1,2 and 3], Distilled water and green coffee extract showed no zone of inhibition against both the bacterial species. While the widest zone of inhibition was displayed by 1% amoxicillin against both A. actinomycetemcomitans and P. gingivalis. For all the antimicrobial agents incorporated in the study the mean zone of inhibition for each bacterium was calculated for analysis [Table/Fig-4,5]. Amoxicillin, when compared to the other antimicrobial agents as well as negative control used in the study showed level of significance against both A. actinomycetemcomitans and P. gingivalis [Table/Fig-4]. Whereas, neither metronidazole nor green coffee extract showed any level of significance against A. actinomycetemcomitans using Mann- Whitney U test. Amoxicillin showed better results against P. gingivalis as compared to metronidazole (specific for anaerobic bacterial Infections). Metronidazole showed level of significance against P. gingivalis.
Zones of inhibition for various anti-microbial agents.
| Antimicrobial Extracts | Microorganisms Tested |
|---|
| A. actinomycetemcomitans | P. gingivalis |
|---|
| Amoxicillin | 42 x 46 | 43 x 48 | 41 x 45 | 22 X 18 | 30 X 24 | 24 X 18 |
| Metronidazole | 16 X 16 | 0 x 0 | 10 X 10 | 18 X 14 | 18 X 16 | 17 X 14 |
| Green coffee extract | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 |
| Distilled Water | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 | 0 x 0 |
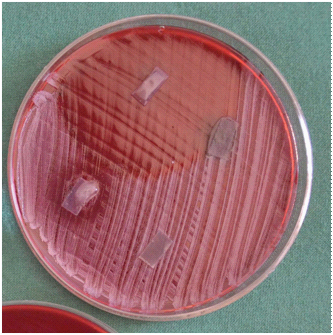
GTR membrane loaded with antimicrobial agents placed on lawn of A. actinomycetemcomitans showing zone of inhibition.
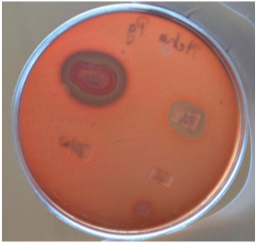
GTR membrane loaded with antimicrobial agents placed on lawn of P. gingivalis showing zone of inhibition.
Pair wise comparisons of four anti-microbial agents with zone of anti-microbial activity of A. actinomycetemcomitans by Mann-Whitney U test.
| Anti-MicrobialAgents | Mean | SD | SE | Sum of Ranks | U-Value | Z-Value | p-value |
|---|
| Amoxicillin | 1947.00 | 110.27 | 63.66 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Metronidazole | 118.67 | 129.02 | 74.49 | 6.00 |
| Amoxicillin | 1947.00 | 110.27 | 63.66 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Green Coffee | 0.00 | 0.00 | 0.00 | 6.00 |
| Amoxicillin | 1947.00 | 110.27 | 63.66 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Distilled Water | 0.00 | 0.00 | 0.00 | 6.00 |
| Metronidazole | 118.67 | 129.02 | 74.49 | 13.50 | 1.50 | −1.3093 | 0.1904 |
| Green Coffee | 0.00 | 0.00 | 0.00 | 7.50 |
| Metronidazole | 118.67 | 129.02 | 74.49 | 13.50 | 1.50 | −1.3093 | 0.1904 |
| Distilled Water | 0.00 | 0.00 | 0.00 | 7.50 |
| Green Coffee | 0.00 | 0.00 | 0.00 | 10.50 | 4.50 | 0.0000 | 1.0000 |
| Distilled Water | 0.00 | 0.00 | 0.00 | 10.50 |
*p<0.05
Pair wise comparisons of four anti-microbial agents with zone of antimicrobial activity of P gingivalis by Mann-Whitney U test.
| Anti-Microbialagents | Mean | SD | SE | Sum of ranks | U-Value | Z-Value | p-value |
|---|
| Amoxicillin | 516.00 | 177.58 | 102.53 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Metronidazole | 259.33 | 25.79 | 14.89 | 6.00 |
| Amoxicillin | 516.00 | 177.58 | 102.53 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Green Coffee | 0.00 | 0.00 | 0.00 | 6.00 |
| Amoxicillin | 516.00 | 177.58 | 102.53 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Distilled Water | 0.00 | 0.00 | 0.00 | 6.00 |
| Metronidazole | 259.33 | 25.79 | 14.89 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Green Coffee | 0.00 | 0.00 | 0.00 | 6.00 |
| Metronidazole | 259.33 | 25.79 | 14.89 | 15.00 | 0.00 | −1.9640 | 0.0495* |
| Distilled Water | 0.00 | 0.00 | 0.00 | 6.00 |
| Green Coffee | 0.00 | 0.00 | 0.00 | 10.50 | 4.50 | 0.0000 | 1.0000 |
| Distilled Water | 0.00 | 0.00 | 0.00 | 10.50 |
*p<0.05
Discussion
Microbial colonization of the GTR membrane adversely affects the treatment outcomes of the periodontal treatment [9]. Attachment of the periodontal pathogens onto the GTR membrane may expedite the recurrence of periodontitis and loss of alveolar bone. Direct Incorporation of the antimicrobial agent In the GTR membrane may allow appropriate Infection control. Previous studies of loading tetracycline and amoxicillin on GTR membrane have shown to drastically reduce the adherence of A. actinomycetemcomitans and Streptococcus mutans [14].
The present study consists of loading minimal concentration of antimicrobial agents onto reconstituted Type-I collagen GTR membranes and evaluating Its antimicrobial efficacy on A. actinomycetemcomitans and P. gingivalis. The loading of GTR membrane with commercially available antimicrobial agents can aid in elimination of locally residing pathogenic bacteria leading to better periodontal treatment outcomes. Usage of such a trivial amount of antimicrobial agents onto GTR membrane can lead to the possible improvement in its clinical implications. The ideology behind the present study was to check the antimicrobial property of various allopathic and herbal antimicrobial agents against periodontogenic pathogens at a minimal concentration of 1%. This is one of its kind studies in which green coffee extract was loaded onto the GTR membrane to evaluate its antimicrobial activity against A. actinomycetemcomitans and P. gingivalis. Amoxicillin belongs to the family of β-Lactam antibiotics [15]. Amoxicillin exhibits its antimicrobial property by inhibiting the cross linkage between linear peptidoglycan polymer chains and hence, preventing the formation of bacterial cell wall synthesis [15]. According to Renvert S et al., there is selective elimination of periodontal pathogens after mechanical debridement in cases of advanced chronic periodontitis [16]. In his study, Renvert S et al., concluded that after mechanical debridement, A. actinomycetemcomitans could not be eliminated while there was significant reduction of P. ginigivalis from the positive sites [16]. Earlier, for eliminating subgingival population of A. actinomycetemcomitan, tetracyclines were used [17]. Markman C et al., determined the release of tetracycline hydrochloride from a cellulose GTR membrane and suggested that it may prevent the colonization of the bacterial species on it [10]. Zarkesh N et al., demonstrated additional gain in clinical attachment level by using tetracycline loaded e-Polytetrafluoroethylene (e PTFE) membrane [12]. However, various studies have been documented highlighting the failure of tetracycline therapy against A. actinomycetemcomintans [18–20]. Hung S-L et al., in a study demonstrated reduced adherence of A. actinomycetemcomitans and S. mutans onto GTR membrane loaded with tetracycline and amoxicillin which shows similar results to the present study [14].
Metronidazole is an antibiotic which belongs to the group, nitroimidazole. It was originally introduced to treat Trichomonas vaginalis infections but is presently used to treat anaerobic and protozoal infectons [21]. Metronidazole mediates its bactericidal action by producing toxic metabolites which result in the DNA breakage of the bacterial cell [21]. In the present study metronidazole when coated over GTR membrane showed better results against P. gingivalis as compared to A. actinomycetemcomitans. A similar study concluded that there was a marked improvement in clinical sites in patients when GTR membrane was surface coated with metronidazole [11]. Dowell P et al., concluded that it also helped in reducing post-operative discomfort to the patient [11]. Various authors reported similar findings following use of metronidazole as a subgingival irrigant [22,23] as well as topical application of sustained release antibiotics [24,25].
The market is presently laden with various popular therapeutic antimicrobial products. The quest for developing herbal remedies with extensive array of antimicrobial properties without the side effects of synthetic medications is still going on for treating periodontal disease [26]. Lately, green coffee extract has received much attention amid all the various herbal products because of its antimicrobial properties against both Gram-positive as well as Gram-negative organisms [27]. There are various components of coffee that have been documented to show antimicrobial properties, these include volatile and nonvolatile organic acids, caffeine, phenols and aromatic compounds. Growth of various Gram-positive microorganisms such as Staphylococcus aureus, Bacillus cereus, Lactobacillus bulgaricus, Streptococus lactis and Streptococcus faecalis and Gram-negative bacteria like Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa are inhibited by a non-volatile organic acid found in coffee i.e., CGA and caffeic acid [27]. An in vitro study recognized antimicrobial activity of green coffee extract against four periodonto-pathogens at a very low concentration. It was observed that Minimal Inhibitory Concentration (MIC) of green coffee extract was 0.2 μg/ml for Prevotella intermedia, P. gingivalis, and A. actinomytemcomitans [28]. Yi TL et al., contrasted the antimicrobial activity of 0.2% chlorhexidine mouthwash and different concentrations of green coffee extract against P. gingivalis, P. intermedia, F. nucleatum and A. actinomycetemcomitans [29]. The results of the study [29] concluded that chlorhexidine showed better results as compared to green coffee extract while, 20% and 15% concentration of green coffee extract showed antimicrobial activity against P. intermedia,P. gingivalis and A. actinomycetemcomitans. The present study showed negative results with green coffee extract, showing no antimicrobial property against both the bacterial species. To further evaluate the efficacy of green coffee extract, higher concentrations of extract should have been tested for the present study.
The present in vitro study was designed to evaluate the efficacy of 1% amoxicillin, 1% metronidazole and 1% green coffee extract loaded onto GTR membrane against A. actinomycetemcomitans and P. gingivalis. Zone of inhibition was measured in order to estimate the antimicrobial action while, incorporating attachment and penetration [30] of bacteria onto the antibacterial agent loaded GTR membrane would have given a better perspective to the present study. As green coffee extract showed negative results hence, higher concentrations of the extract should have been tested for their efficacy by loading onto the GTR membrane. Various other plant origin antibacterial agents should be tested and incorporated into non-surgical as well as surgical periodontal therapy in order to avoid the side effects, due to commercially available synthetic antimicrobial agents.
Conclusion
Various studies have documented that GTR membrane is one of the successful methods for periodontal regeneration but it is prone for bacterial contamination thereby, impeding periodontal regeneration. Systemic administration of antibiotics may give rise to drug resistance as well as side effects. Therefore, loading of antimicrobial agents onto the GTR membrane may prevent its bacterial colonization. In the present study, 1% amoxicillin has shown significant antimicrobial effect against both aerobic and anaerobic bacteria. From the present study, it can be concluded that usage of commercially available antimicrobial agents in such a low concentration can effectively reduce the number of periodontal pathogens colonizing the GTR membrane.
*p<0.05
*p<0.05
[1]. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE, Defining the normal bacterial flora of the oral cavityJ Clin Microbiol 2005 43:5721-32. [Google Scholar]
[2]. Faveri M, Figueiredo LC, Duarte PM, Mestnik MJ, Mayer MP, Feres M, Microbiological profile of untreated subjects with localized aggressive periodontitisJ Clin Periodontol 2009 36:739-49. [Google Scholar]
[3]. Miura M, Hamachi T, Fujise O, Maeda K, The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitisJ Periodontal Res 2005 40:147-52. [Google Scholar]
[4]. Ishikawa I, Kawashima Y, Oda S, Iwata T, Arakawa S, Three case reports of aggressive periodontitis associated with Porphyromonas gingivalis in younger patientsJ Periodontal Res 2002 37:324-32. [Google Scholar]
[5]. Preshaw PM, Taylor JJ, How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis?J Clin Periodontol 2011 38:60-84. [Google Scholar]
[6]. Stahl SS, Froum S, Tarnow D, Human histologic responses to guided tissue regenerative techniques in intrabony lesions. Case reports on 9 sitesJ Clin Periodontol 1990 17:191-98. [Google Scholar]
[7]. Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J, New attachment formation in the human periodontium by guided tissue regeneration. Case reportsJ Clin Periodontol 1986 13:604-16. [Google Scholar]
[8]. Machtei EE, Cho M-I, Dunford R, Norderyd J, Zambon JJ, Genco RJ, Clinical, microbiological, and histological factors which influence the success of regenerative periodontal therapyJ Periodontol 1994 65:154-61. [Google Scholar]
[9]. Nowzari H, MacDonald ES, Flynn J, London RM, Morrison JL, Slots J, The dynamics of microbial colonization of barrier membranes for guided tissue regenerationJ Periodontol 1996 67:694-702. [Google Scholar]
[10]. Markman C, Francakanzza SE, Novaes AB, JrNovaes AB, Slow release of tetracycline hydrochloride from a cellulose membrane used in guided tissue regenerationJ Periodontol 1995 66:978-83. [Google Scholar]
[11]. Dowell P, Al-Arrayed F, Adam S, Moran J, A comparative clinical study: The use of human type I collagen with and without the addition of metronidazole in the GTR method of treatment of periodontal diseaseJ Clin Periodontol 1995 22:543-49. [Google Scholar]
[12]. Zarkesh N, Nowzari H, Morrison JL, Slots J, Tetracycline-coated polytetra- flouroethylene barrier membranes in treatment of intraosseous periodontal lesionsJ Periodontol 1999 70:1088-16. [Google Scholar]
[13]. Siddiqui HH, Safety of herbal drugs - An overviewDrugs News Views 1993 1:7-10. [Google Scholar]
[14]. Hung S-L, Ling Y-W, Wang Y-H, Chen Y-T, Su C-Y, Ling L-J, Permeability of Streptococcus mutans and Actinobacillus actinomycetemcomitans through guided tissue regeneration membranes and their effect on attachment of periodontal ligament cellsJ Periodontol 2002 73:843-51. [Google Scholar]
[15]. Handsfield HH, Clark H, Wallace JF, Holmes KK, Turck M, Amoxicillin, a new penicillin antibioticAntimicrob Agents Chemother 1973 3:262-65. [Google Scholar]
[16]. Renvert S, Wikström M, Dahlén G, Slots J, Egelberg J, Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pocketsClin Periodontol 1990 17:345-50. [Google Scholar]
[17]. Slots J, Rams TE, Antibiotics in periodontal therapy: Advantages and disadvantagesClin Periodontol 1990 17:479-93. [Google Scholar]
[18]. Goené RJ, Winkel EG, Abbas F, Rodenburg JP, van Winkelhoff AJ, de Graaff J, Microbiology in diagnosis and treatment of severe periodontitis. A report of four casesJ Periodontol 1990 61:61-64. [Google Scholar]
[19]. Mandell EL, Socransky SS, Microbiological and clinical effects of surgery plus doxycycline on juvenile periodontitisJ Periodontol 1988 59:373-79. [Google Scholar]
[20]. de Graaff J, van Winkelhoff AJ, Goené RJ, The role of Actinobacillus actinomycetemcomitans in periodontal diseasesInfection 1989 17:269-71. [Google Scholar]
[21]. Lamp KC, Freeman CD, Klutman NE, Lacy MK, Pharmacokinetics and pharmacodynamics of the nitoimidazole antimicrobialsClin Pharmacokinet 1999 36:353-73. [Google Scholar]
[22]. Rosling BG, Slots J, Webber RL, Christersson LA, Genco RJ, Microbiological and clinical effects of topical subgingival antimicrobial treatment on human periodontal diseaseJ Clin Periodontol 1983 10:487-514. [Google Scholar]
[23]. MacAlpine R, Magnusson I, Kiger R, Crigger M, Garrett S, Egelberg J, Antimicrobial irrigation of deep pockets to supplement oral hygiene instruction and root debridement. I. Bi-weekly irrigationJ Clin Periodontol 1985 12:568-77. [Google Scholar]
[24]. Pedrazzoli V, Kilian M, Karring T, Comparative clinical and microbiological effects of topical subgingival application of metronidazole 25% dental gel and scaling in the treatment of adult periodontitisJ Clin Periodontol 1992 19:715-22. [Google Scholar]
[25]. Ainamo J, Lie T, Ellingsen BH, Hansen BF, Johansson LA, Karring T, Clinical responses to subgingival application of a metronidazole 25% gel compared to the effect of subgingival scaling in adult periodontitisJ Clin Periodontol 1992 19:723-29. [Google Scholar]
[26]. Walker CB, The acquisition of antibiotic resistance in the periodontal microfloraJ Periodontol 2000 1996 10:79-88. [Google Scholar]
[27]. Fardiaz S, Antimicrobial activity of coffee (Coffea robusta) extractASEA Food J. 1995 10:103-06. [Google Scholar]
[28]. Bharath N, Sowmya NK, Mehta DS, Determination of antibacterial activity of green coffee bean extracts on periodontogenic bacteria like Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans: An in-vitro studyContemp Clin Dent 2015 6:166-69. [Google Scholar]
[29]. Yi TL, Shah M, Raulji D, Dave D, Comparative evaluation of antimicrobial efficacy of coffee extract and 0.2% chlorhexidine mouthwash on the periodontal pathogens Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans: An in-vitro studyAdv Hum Biol 2016 6:99-103. [Google Scholar]
[30]. Cheng CF, Lee YY, Chi LY, Chen YT, Hung SL, Ling LJ, Bacterial penetration through antibiotic-loaded guided tissue regeneration membranesJ Periodontol 2009 80:1471-78. [Google Scholar]