Orbital Myiasis with Scalp Pediculosis and Buccal Abscess–An Uncommon Presentation
Nidhi Kaeley1, Rajeev Mohan Kaushik2, Richa Rajput3, Renu Dhasmana4, Anurag Bhargava5
1 Assistant Professor, Department of Internal Medicine, Himalayan Institute of Hospital Trust, Dehradun, Uttarakhand, India.
2 Professor, Department of Internal Medicine, Himalayan Institute of Hospital Trust, Dehradun, Uttarakhand, India.
3 Resident, Department of Internal Medicine, Himalayan Institute of Hospital Trust, Dehradun, Uttarakhand, India.
4 Professor, Department of Ophthalmology, Himalayan Institute of Hospital Trust, Dehradun, Uttarakhand, India.
5 Professor, Department of Internal Medicine, Himalayan Institute of Hospital Trust, Dehradun, Uttarakhand, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Nidhi Kaeley, E-30 Bungalow Road Kamla Nagar, Delhi-110007, India.
E-mail: drnidhi_kaeley@yahoo.com
We present a case of severe orbital myiasis caused by Osteris ovis, also known as sheep nasal botfly which is an uncommon manifestation of maggot infestation. Our patient was successfully treated with oral as well as topical ivermectin. The experience of ivermectin as an anti-parasitic agent in the treatment of orbital myiasis, although a known entity, is still limited among medical professionals including ophthalmologists. Thus, we would like to highlight the role of oral ivermectin as an anti-parasitic agent in the treatment of orbital myiasis facilitating the removal of maggots; thus, precluding the need for exploratory surgery.
Ivermectin, Maggot infestation, Osteris ovis
Case Report
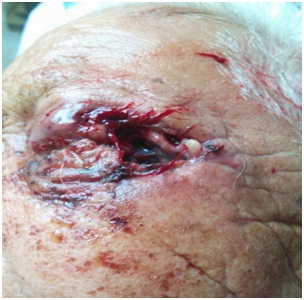
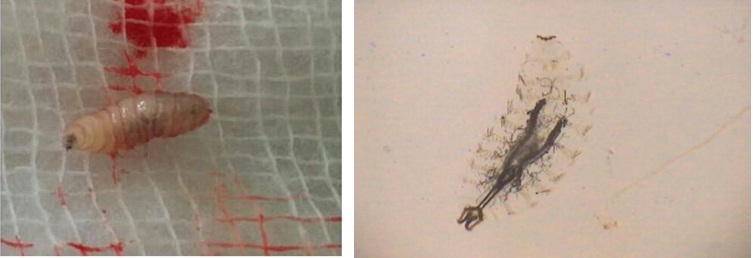
A 90-year-old elderly female, resident of Kotdwar, presented to us in the emergency with altered sensorium and left orbital wound filled with hatching maggots. She belonged to an army background and had been living alone for the last 15 years. She had a fractured right tibia with mal-union and leg deformity such that the ambulation was restricted to walking with the support of a walker. She had a poor self-care. Vision in the right eye was lost 8 years back due to pterygium. She developed chalazion in the left upper eyelid 2 years back, which was asymptomatic with mild itching. The itching increased over the last one week and the old lady, out of irritation, scratched the lid with a dirty safety pin. Her upper lid swelled up over the next few hours, followed by the development of peri-orbital puffiness, redness and tenderness over lateral canthus, nasal bridge and forehead. Over the period of one day, the chalazion burst out with exudation of dark brownish black colored thick fluid and clots along with the frank yellow colored pus, followed by expulsion of maggots from the pus point of the wound at lateral canthus of the left eye [Table/Fig-1]. On examination, she was irritable, confused and afebrile. Her pulse rate was 46/min and BP 124/80mmHg. There was no sensory or motor neurological deficit or any other systemic examination abnormality for age. A left orbital wound measuring 4x5cm was present involving lateral canthus. Right eye had copious amount of secretions and debris, short palpebral fissure due to ciccatrization and fusion of upper and lower eyelids with no vision. Scalp pediculosis was also present. There was poor oral hygiene and loss of teeth in both upper and lower jaws. She also had buccal abscess. Right leg deformity was also noted. Investigations revealed mild anaemia (Hemoglobin-9g/dl), leucocytosis (TLC-14000/cumm, DLC-neutrophils-75%, lymphocytes-20%, eosinophil-4%) and raised serum creatinine level (1.95mg/dl) with normal sodium and potassium levels. The raised creatinine was due to sepsis induced acute renal failure. Her HIV status along with other viral markers was negative. There was no evidence of meningitis on Cerebrospinal fluid (CSF) examination. MRI brain and orbits reported ill defined heterogeneously hyperintense mass at the lateral canthus of left eye with mild intra-orbital extra-conal extension, chronic infarct left MCA territory, peri-ventricular white matter ischemic changes and age related cerebral atrophy. She was stabilized in emergency and was given first aid. Wound was cleaned; 15 live maggots were removed with forceps and hypertonic saline was instilled [Table/Fig-2]. The patient was given a single dose of tablet ivermectin (200mg/Kg) and ivermectin eyedrops 1% (w/v) were prepared which were instilled in the wound (2 drops, 6th hourly). Following this stat dose, 12 dead maggots were extracted over the next two days respectively and were sent for entomological examination. There were no maggots in the wound 3 days after the start of ivermectin therapy. She was also given tetanus toxoid prophylaxis, iv antibiotic – ceftriaxone 1gm iv twice daily for 5 days in view of secondary bacterial infection, iv analgesics, fluids and other supportive care. The patient’s general condition improved, irritability decreased over the next two days with normalization of kidney function tests. Wound in the buccal cavity was explored under general anesthesia and around 20ml of pus was drained from the buccal cavity. The patient was discharged in a stable condition with minimal restoration of vision in left eye to the extent of perception of light. The larvae were found to be of well-known sheep nasal botfly, the Oestris Ovis.
Left lateral canthus orbital myiasis.
Spindle shaped larva of Oestrus ovis with a pair of hooklets at anterior region.
Discussion
Human myiasis is the invasion of human tissue by larvae from the order Diptera [1]. Orbital myiasis, or maggot infestation, is a rare condition with very few reports with reported incidence of 5% cases, published worldwide. Members of cyclorhabid, mainly Sarcophaga and OestridaeDiptera, may produce myiasis in countries with poor hygiene and abundant flies [1]. The genus Oestrus includes the cosmopolitan well-known sheep nasal botfly, the Oestris Ovis [2]. Other species causing furuncular and wound myiasis are Dermatobia hominis, Cordylobia anthropophaga, Wohlfahrtia magnifica, Chrysomyia bezziana, and Cochliomyia hominivorax world wide. Ocular myiasis in humans was first reported by Keyt in 1900. The first case from India was described by Elliot in 1910 [3]. This condition has been reported from Libya, Afghanistan, Oman, Africa, Iran and in India from Tamil Nadu, Northern India and Western Uttar Pradesh. Management of orbital myiasis ranges from simple manual removal of the maggots to destructive surgeries of the globe and orbit [4]. With the advent of the broad-spectrum anti-parasitic drug, ivermectin, there is now a safe and non-invasive means of tackling maggots, especially those buried deep in the orbital tissues [5].
Cases of severe myiasis are usually found in patients with low socio-economic status. History of alcohol use, social isolation, and poverty is frequently found in these patients. Although maggot infestations are common in the tropics, only between 5 and 14% of all cases involve the ocular tissues and most ophthalmologists have little experience with this condition [6,7]. Maggots are the larvae of those dipteran flies which need a host for completion of their life cycle. The definitive hosts for maggots are goats and sheep. Man is an accidental host, and trauma is an important risk factor. Eggs are deposited by the flies in the host within 8 to 24 hours. This time is dependent on the species and environmental conditions. The larva formed starts feeding on the dead and infected tissues and leaves the host. After isolating from the host, they continue growing in an isolated place. Thereby it gets converted into a pupa with a dark exoskeleton. It takes one to three weeks for a pupa to become an adult fly [8]. Myiasis cases tend to occur in the spring and summer. Our patient presented in the month of May with a recent lid injury. Successful treatment of orbital myiasis depends on the degree of involvement at the time of the initial evaluation and the severity of underlying pre-disposing conditions. Historically, treatment entails mechanical extraction of the maggots, and it has been suggested that, there is no anti-parasitic therapy that will dislodge the larvae [9]. In addition, cases of severe orbital myiasis require prompt surgical debridement to prevent the risk of involving vital structures. More recently, the use of anti-parasitic therapy has been suggested as a potential adjunctive treatment. Ivermectin is a semi-synthetic macrocyclic lactone that has been demonstrated to have activity against Hominivorax, Dermatobia hominis, Oestris Ovis, Wuchereria bancrofti, Wuchereria malayi, Onchocerca volvulus, and Loa loa, as well as ectoparasites, such as Sarcoptes scabies, Pediculus humanus, Demodex folliculorum, and Cheyletiella species. Ivermectin has, therefore, become an important alternative for the treatment of patients with different forms of ectoparasite infestations, such as scabies, head lice, demodicidosis and myiasis [10].
Scalp pediculosis is a pre-disposing factor for scalp myiasis [7], although it did not occur in our patient as she had the habit of wearing scarf. In view of the massive, mixed parasitic infestation and the persistence of deeply buried maggots, it was decided to treat the patient with ivermectin so as to prevent exenteration. The oral dose of ivermectin is 200μg/kg body weight. Ivermectin acts by inhibiting neurotransmission as it causes hyperpolarization of the neurons and myocytes of invertebrates. Ivermectin is known to have a very high safety profile in humans [11]. Maggots have been reported to have anti-bacterial activity [12].Thus, systemic antibiotics may not be required to prevent secondary pyogenic infection but antibiotics were given in our patient as she also had buccal abscess; thus, preventing bacterial superinfection. Maintenance of personal hygiene is an essential preventive measure to avoid maggot infestation.
Conclusion
Ivermectin is useful in managing severe orbital myiasis and may prevent the need of extensive surgical debridement if given early. The knowledge of usage of ivermectin as an anti-parasitic drug is still limited among medicine fraternity which is highlighted by this case.
[1]. Da Silva BB, Borges US, Pimentel ICC, Human vaginal myiasis caused by Cochliomyia hominivoraxInt J Gynaecol Obstet 2005 89:152-53. [Google Scholar]
[2]. Goel H, Tangri R, Kaur R, Jain J, Two case reports of ophthalmomyiasis externa caused by Oestris Ovis larvaeAnn Trop Med Public Health 2012 5:549-50. [Google Scholar]
[3]. Sivaramasubramanyam P, Sadanand AV, OpthalmomyiasisBr J Ophthalmol 1968 52:52-65. [Google Scholar]
[4]. Gunalp I, Gunduz K, Duruk K, Orbital exenteration: A review of 429 casesInt Ophthalmol 1995 19:177-84. [Google Scholar]
[5]. Osorio J, Moncada L, Molano A, Valderrama S, Gualtero S, Franco-Paredes C, Role of Ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivoraxClin Infect Dis 2006 43:e57-59. [Google Scholar]
[6]. Wilhelmus KR, Myiasis palpebrumAm J Ophthalmol 1986 101:496-8. [Google Scholar]
[7]. Hemmings SC, Matthews KJ, Alexander J, Human myiasis in western Jamaica: Five years after the implementation of a screwworm eradication programmeWest Indian Med J 2007 56:341-45. [Google Scholar]
[8]. Manfrim AM, Cury A, Demeneghi P, Jotz G, Roithmann R, Nasal myiasis: Case report and literature reviewInt Arch Otorhinolaryngol 2007 11(1):74-79. [Google Scholar]
[9]. Sigauke E, Beebe W, Gander R, Cavuoti D, Southern P, Case report: Ophthalmomyiasis externa in Dallas County, TexasAm J Trop Med Hyg 2003 68:46-47. [Google Scholar]
[10]. Radmanesh M, Khataminia G, Eliasi P, Korai MK, Ebrahimni A, Crysomyia bezziana-infested basal cell carcinoma destroying the eyeInt J Dermatol 2000 39:455-62. [Google Scholar]
[11]. Lima WS, Malacco MA, Bordin EL, Oliveira EL, Evaluation of the prophylactic effect and curative efficacy of fipronil 1% pour on (Topline) on post-castration scrotal myiasis caused by Cochliomyia hominivorax in cattleVet Parasitol 2004 125:373-77. [Google Scholar]
[12]. Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjectsJ Clin Pharmacol 2002 42:1122-33. [Google Scholar]