Versatility of Tongue Flaps for Closure of Palatal Defects- Case Report
Vishnu Mohan1, Roopesh U Nair2, Arjun Madhu Usha3
1 Professor, Department of Oral and Maxillo Facial Surgery, Azeezia College of Dental Science and Research, Meeyyanoor, Kerala, India.
2 Senior Lecturer, Department of Oral and Maxillo Facial Surgery, Azeezia College of Dental Science and Research, Meeyyanoor, Kerala, India.
3 Postgraduate Student, Department of Oral and Maxillo Facial Surgery, Azeezia College of Dental Science and Research, Meeyyanoor, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Arjun Madhu Usha, Thushara Miniestate Road, Meppukkada, Malayinkil, Kerala, India.
E-mail: drarjunmu@gmail.com
Palatal defects following congenital anomalies, traumatic injuries, benign and malignant pathologies frequently require resection and reconstruction. Reconstruction of these defects is challenging and complex due to the amount of tissue left for primary closure after excision, compromised vasculature as on repaired cleft palate and limited pedicled flaps around the lesion. Tongue flap though doesn’t fulfil all the ideal requirements of a flap, however because of its flexibility, good blood supply and position it can be considered as the best among other flaps for reconstruction of oral and palatal defects. In this article we describe two different cases in which tongue flap was used to reconstruct palatal defects, one an oroantral communication secondary to a tumour excision and the other an oro-nasal fistula secondary to cleft palate repair.
Cleft palate, Oroantral fistula, Oro-nasal fistula, Pedicled flap
Case-1
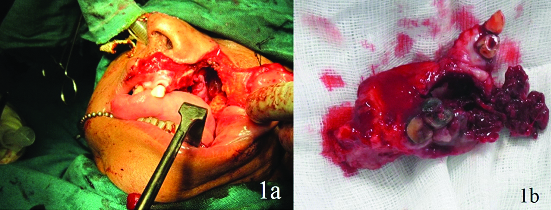
A 72-year-old female patient reported to the Department of Oral and Maxillofacial Surgery, with a chief complaint of growth over the upper left back tooth region since one month. She noticed the growth as a small mass that progressively enlarged in size and it was associated with pain from the past one week. The pain was intermittent, moderate in intensity and relieved by analgesics. On examination, there was no obvious facial swelling. Intraoral examination revealed a swelling of 4x3 cm in diameter soft in consistency with irregular edges around maxillary left second and third molars covering up to the middle thirds of crown of these teeth. The growth showed red and white areas and was tender on percussion. The patient was advised for routine blood investigation and Computed Tomography (CT) scan. CT report showed a lesion with greatest dimension of 7x5 cm involving the left posterior maxilla and maxillary sinus with ill-defined granular bone periphery. Internally the lesion showed evidence of large hypodense and hyperdense areas with thin trabecular pattern and thin separations within the lesion. Roots of second molars showed areas of resorption. The medial, superior and posterior lateral walls of the left maxillary sinus were intact. An incisional biopsy was taken from the first molar region and was sent for histopathological examination. The microscopic examination showed parakeratinized stratified squamous epithelium which appeared hyperplastic and underlying connective tissue showed dense chronic inflammatory cell infiltrate, chiefly lymphocytes. The deeper connective tissue showed dispersed multinuclear giant cells in association with vesiculated mononuclear cells with moderate vascularity. The histopathological report confirmed the mass as central giant cell granuloma. A subtotal maxillectomy was planned for the patient. Written informed consent was obtained from the patient. Patient was prepared and draped under orotracheal intubation. Weber Ferguson incision was made through the skin and subcutaneous tissue. Upper lip was divided through its full thickness upto gingival labial sulcus and incision was extended sublabially along the mucobuccal fold upto the tuberosity. The subciliary component was extended preseptally. A subperiosteal cheek flap was then elevated from the maxilla. The maxilla was fully exposed and a subtotal maxillectomy was performed removing the tumour in toto [Table/Fig-1]. As the primary closure of the defect couldn’t be made during the surgery, a preoperatively fabricated obturator was placed and stabilized to the cheek flap using 3-0 ethilon suture. Postoperative period of the patient was uneventful. Patient was kept under observation for one year.
a) Weber Ferguson incision; b) Subtotal maxillectomy.
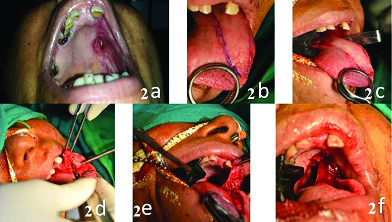
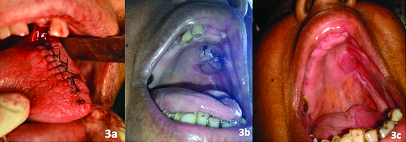
One year follow up showed a reduction in the size of defect to about 2x3 cm, then a posteriorly based tongue flap was planned to close the oroantral fistula. Written informed consent was taken from the patient. Patient was prepared and draped under nasotracheal intubation. De-epithelization was done around the oroantral fistula. A posteriorly based tongue flap was raised and sutured to the defect. The patient was advised Ryle’s tube feeding and intermaxillary fixation for two weeks. After two weeks vascularity of the flap was checked and the flap was divided from the donor site. Healing of the surgical site was satisfactory [Table/Fig-2]. During the first year follow up, the patient showed no signs of recurrence of oroantral fistula [Table/Fig-3].
a) One year postoperative after tumour excision; b) Outline of the incision; c) Incision in place; d) Dissecting posterior based tongue flap; e) Checking for adequate length; f) Sutured flap in place.
a) Primary closure of tongue; b) Healing after one month; c) Healing after one year.
Case-2
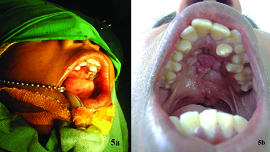
A 14-year-old female patient reported to our department with chief complaint of difficulty in speech and regurgitation of food and fluids through the nose. Parents reported that due to improper speech, the patient was under depression. Parents gave a history of primary cleft palate repair at the age of one and half year for attaining normal speech and swallowing. On examination, an elliptical communication of 2x1.5 cm extending from the junction of hard and soft palate till the nasal cavity with a lining was seen. Surgical scar of operated cleft palate was seen extending from the fistula till the uvula. She also had nasal regurgitation and difficulty in speech. Based on history and clinical examination a diagnosis of secondary deformity of cleft palate repair-oro nasal fistula was made. Patient was then planned for fistula closure using a tongue flap. Written informed consent was obtained from the parents.
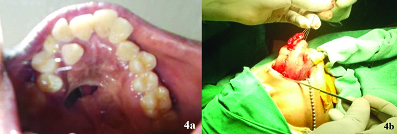
The patient was prepared and draped under orotracheal intubation. Dingman retractor was placed to visualize the fistula. The edges of the fistula were injected with 2% lignocaine and 1:200000 adrenaline. An incision was placed around the fistula and the nasal mucosal layer was dissected and raised, tension free closure of the nasal layer was achieved using resorbable sutures. A pedicled anteriorly based tongue flap was designed based on the defect and amount of rotation needed, which was then dissected, elevated and sutured to the edges of the incised palatal mucosa to create an oral layer [Table/Fig-4]. The patient was placed on inter maxillary fixation for two weeks with feeding through the Ryle’s tube. After two weeks the flap was checked for its vascularity. Once the vascularity was confirmed, flap was divided from the donor site. The patient was reviewed after one more week and healing of the site was found satisfactory. The patient was then advised to consult a speech therapist to improve her speech. The patient was on regular follow up during the past six months and her speech improved considerably with no signs of recurrence of fistula [Table/Fig-5].
a) Oro nasal fistula secondary to cleft palate repair; b) Raising of anterior based tongue flap.
a) Anterior based tongue flap in place; b) Healing after six months.
Discussion
The tongue flap was first described by Eisenberg for intraoral defects. Later on, Lexer used a lateral based tongue flap in 1909 for reconstruction of defects of retro molar trigon and tonsilar area [1,2]. Guerrero-Santos J, Altamirano JT [3] popularized this technique. The tongue is an excellent donor site for soft tissue defects of the oral cavity, due to its flexibility, position and abundant vascularity [2]. The vascularity of the tongue is through the lingual artery that gives off four branches supra hyoid, dorsal lingual, deep lingual and sublingual artery along with this there is extensive anastomotic network with the contralateral side. Various clinical application of tongue flap includes reconstruction of oral defects, closure of oro- antral fistula, alveolar clefts, oral sub mucous fibrosis, upper and lower lip reconstruction and hard and soft palate defects [4–6].
The reconstruction of palatal defects following various resection and reconstructive surgeries like cleft palate, traumatic, benign and malignant pathologies, oronasal and oroantral communication and fistulas are often complex and challenging. The primary closure of these defects is very difficult due to the less elasticity of the palatal tissues, mucosal atrophy and scar tissues from primary and secondary surgeries [7].
For defects that cannot be closed primarily, a variety of surgical and prosthetic techniques are used, which includes local flaps (buccal advancement flaps, buccal fat pad, naso labial flaps, palatal flaps, tongue flaps) tubed pedicled flaps from abdomen, arm, neck or cervicothoracic region, free nonvascularized grafts such as dermis or conchal cartilage [4,5,8,9]. The Facial Artery Musculomucosal (FAMM) was introduced by Pribaz J et al., for moderate size defects of anterior palate with promising results [10]. Cole P et al., used decellularized human dermal matrix for repair of recurrent oronasal fistula and concluded that the adjacent placement of intramucosal decellularized dermal graft is effective for use in closure of recurrent oronasal fistulas [11].
In both the above described cases tongue flap was considered as a better option than other flaps owing to its good vascular supply, good reach to the defect site, ability to provide adequate tissue bulk, decreased donor site morbidity and increased success rate. For normal healing the replaced tissue should be structurally and functionally similar to the lost tissue. Similarly, in oral cavity the replacement of tissues to compensate for the defect should be as similar as possible. The tongue flap will provide a similar tissue replacement of the palatal defects with a mucosal covering.
Tongue flap has been a work horse for difficult palatal fistula with shortage of tissue and the success rate of the tongue flap has been reported varying from 85% to 95.5% [12]. The commonly used tongue flap designs are anterior based flap, posterior based flap, central island flap, median flap, lateral flap and ventral surface flap. Following precautions has to be taken while raising a tongue flap, length of the flap should be sufficient enough to avoid tension in the flap, principal gustatory papillae should be avoided from the flap, tip of the tongue should be preserved as much as possible and flap should have adequate thickness and should contain mucosa and sub adjacent muscle. Tongue flaps are not commonly used due to the fear of alteration in speech, articulation problems, Postoperative oedema that can compromise the airway and need for second surgeries to divide and debulk the flap. Complications of the procedure include haematoma formation that can compress the pedicle leading to necrosis of the flap, dehiscence and temporary loss of tongue sensation and alteration in taste perception.
Conclusion
In the first case of oroantral fistula secondary to subtotal maxillectomy, even though there was no bone to support still the flap had successfully taken up, that is because of the versatility and rich blood supply of the tongue flap. In the second case we could create a good nasal layer and a good recipient bed for the tongue flap so that the fistula was closed and nasal regurgitation was fully treated. The nasal twang of the patient improved with the speech therapy.
Considering all the advantages and the results that we got, we recommend the reader to consider tongue flap as an ideal flap for reconstruction of intraoral soft tissue defects especially palatal defects.
[1]. Komisar A, The applications of tongue flaps in head and neck surgeryBull N Y Acad Med 1986 62(8):847-53. [Google Scholar]
[2]. Bracka A, The blood supply of the dorsal tongue flapsBr J Plastic Surg 1981 34(4):379-84. [Google Scholar]
[3]. Guerrero-Santos J, Altamirano JT, The use of lingual flaps in repair of fistulas of the hard palatePlast Reconstr Surg 1966 38:123-28. [Google Scholar]
[4]. Deshmukh A, Kannan S, Thakkar P, Chaukar D, Yadav P, D Cruz A, Tongue flap revisitedJournal of Cancer Research and Therapeutics 2013 9(2):215-18. [Google Scholar]
[5]. Sathish MS, Krishnan G, Rai YS, Desai A, The versatility of the tongue flap in the closure of palatal fistulaCraniomaxillofacial Trauma and Reconstruction 2012 5:145-60. [Google Scholar]
[6]. Mahajan RK, Chhajlani R, Ghildiyal HC, Role of tongue flap in palatal fistula repair: A series of 41 casesIndian J Plast Surg 2014 47(2):210-15. [Google Scholar]
[7]. Rahpeyma A, Khajehahmadi S, Posteriorly based lateral tongue flap for reconstruction of large palatal-alveolar fistulas in cleft patientsAnn Maxillofac Surg 2015 5(2):174-78. [Google Scholar]
[8]. Oberna F, Takácsi-Nagy Z, Réthy A, Pólus K, Kásler M, Buccal mucosal transposition flap for reconstruction of oropharyngealeoral cavity defects: An analysis of six casesOral Surg Oral Med Oral Pathol Oral Radiol Endod 2005 99:550-53. [Google Scholar]
[9]. Hao SP, Reconstruction of oral defects with the pedicled buccal fat pad flapOtolaryngo Head Neck Surg 2000 122(6):863-67. [Google Scholar]
[10]. Pribaz J, Stephens W, Crespo L, Gifford G, A new intraoral flap: Facial artery musculomucosal (FAMM) flapPlast Reconstr Surg 1992 90:421-29. [Google Scholar]
[11]. Cole P, Horn TW, Thaller S, The use of decellularized dermal grafting (AlloDerm) in persistent oro-nasal fistulas after tertiary cleft palate repairJ Craniofac Surg 2006 17:636-41. [Google Scholar]
[12]. Murthy J, Descriptive study of management of palatal fistula in one hundred and ninety-four cleft individualsIndian J Plast Surg 2011 44:41-46. [Google Scholar]