Amelanotic Melanoma Arising in Filarial Leg: A Report of a Rare Case
Mamita Nayak1, Susama Patra2, Susanta Meher3, Prakash Kumar Sasmal4
1 Senior Resident, Department of Pathology, AIIMS, BBSR, Bhubaneswar, Odisha, India.
2 Additional Professor, Department of Pathology, AIIMS, BBSR, Bhubaneswar, Odisha, India.
3 Senior Resident, Department of Surgery, AIIMS, BBSR, Bhubaneswar, Odisha, India.
4 Associate Professor, Department of Surgery, AIIMS, BBSR, Bhubaneswar, Odisha, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Susama Patra, Additional Professor, Department of Pathology, AIIMS, BBSR, Bhubaneswar-751019, Odisha, India.
E-mail: wususama@gmail.com
Amelanotic melanoma arising on chronic lymphoedema has not been reported earlier. We reported a case of amelanotic melanoma of right leg developing in a background of chronic lymphoedema of filarial origin in an elderly male of 60 years, who underwent wide local excision of the lesion followed by skin grafting for the same. A histopathological diagnosis of amelanotic melanoma was made. The patient was on follow up when he developed brain metastasis within a period of nine months for which he was operated and is on regular follow up now. We believe this as the first case report of this unusual combination.
Filariasis, Lymphoedema, Lower extremities
Case Report
A 60-year-old male farmer had bilateral filarial lymphoedema of both legs for last 30 years. He presented with the complaint of foul smelling multiple ulcero proliferative lesions encircling the right ankle region from three months duration. To start with there was a small nodular growth in the same site for one and half years back, which was gradually increasing in size and ulcerated with serosanguinous discharge. It was managed conservatively in another hospital with dressing and oral antibiotics. The lesion progressed to a large fungating and multinodular mass which was foul smelling. Some of the nodules showed surface ulceration with oozing.
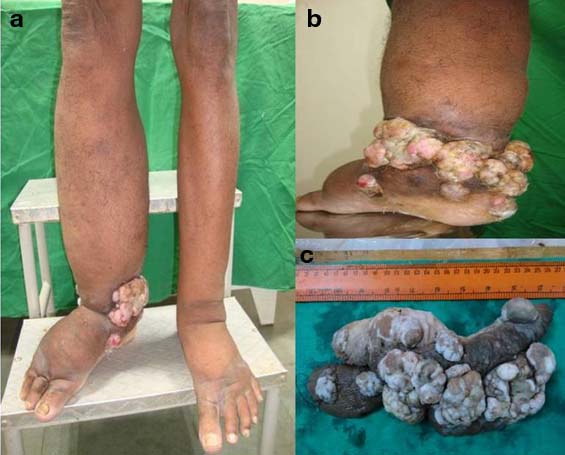
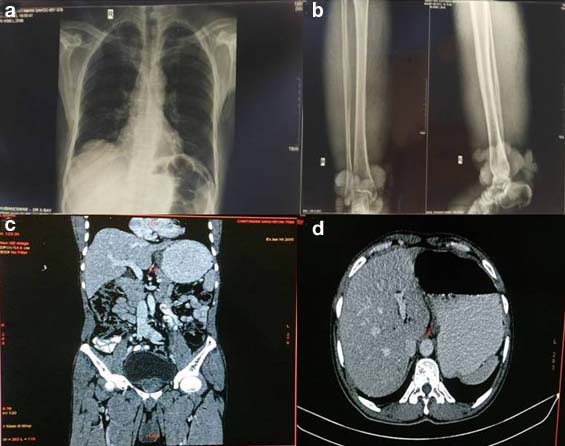
On physical examination both limbs were markedly oedematous but oedema was more in the right lower limb, skin of both legs were markedly thickened with a circumferential nodular mass measuring around 25 cm located above the right ankle region [Table/Fig-1a-c]. Inguinal lymphnodes of both sides were not palpable. Laboratory investigations revealed peripheral eosinophilia with absolute eosinophilic count of 1.89x103 cells per microliter, increased Erythrocyte Sedimentation Rate (ESR) (86 mm in first hour) and normal fasting blood sugar (86 mg/dl). The serological tests for HBsAg, HCV and HIV were negative. Liver function test was normal. No microfilariae was found on peripheral smear. Fluorescent antibody test for Wuchereria bancrofti and Brugia malayi antigens were negative. Culture report from the wound discharge grew Edwardsiella and Enterococcus, which were sensitive to Cefixime, Ceftazidime, Levofloxacin, Ciprofloxacin and Tazobactam-Piperacillin. Chest X-ray was normal. X-ray of right lower limb showed no involvement of underlying bone. Computed Tomography (CT) scan of whole body and abdomen showed no distant metastasis [Table/Fig-2a-d]. He had a family history of filariasis of his mother and belonged to an endemic region of filariasis. The patient had undergone surgery for hydrocele which was of filarial origin 10 years back for which no details were available. Incisional biopsy from the circumferential growth was obtained.
Clinical images: a,b) Swollen limbs with the fungating mass on right lower limb; c) Resected skin with tumour.
Radiological images: a) Chest X-ray was normal; b) X-ray of right lower limb showed no involvement of underlying bone; c) CT scan of whole body shows no distant metastasis; d) Axial CT of abdomen showing no evidence of periportal lymphadenopathy or liver metastasis.
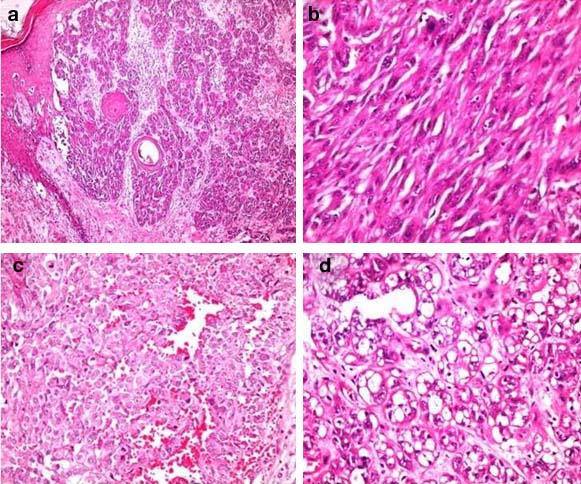
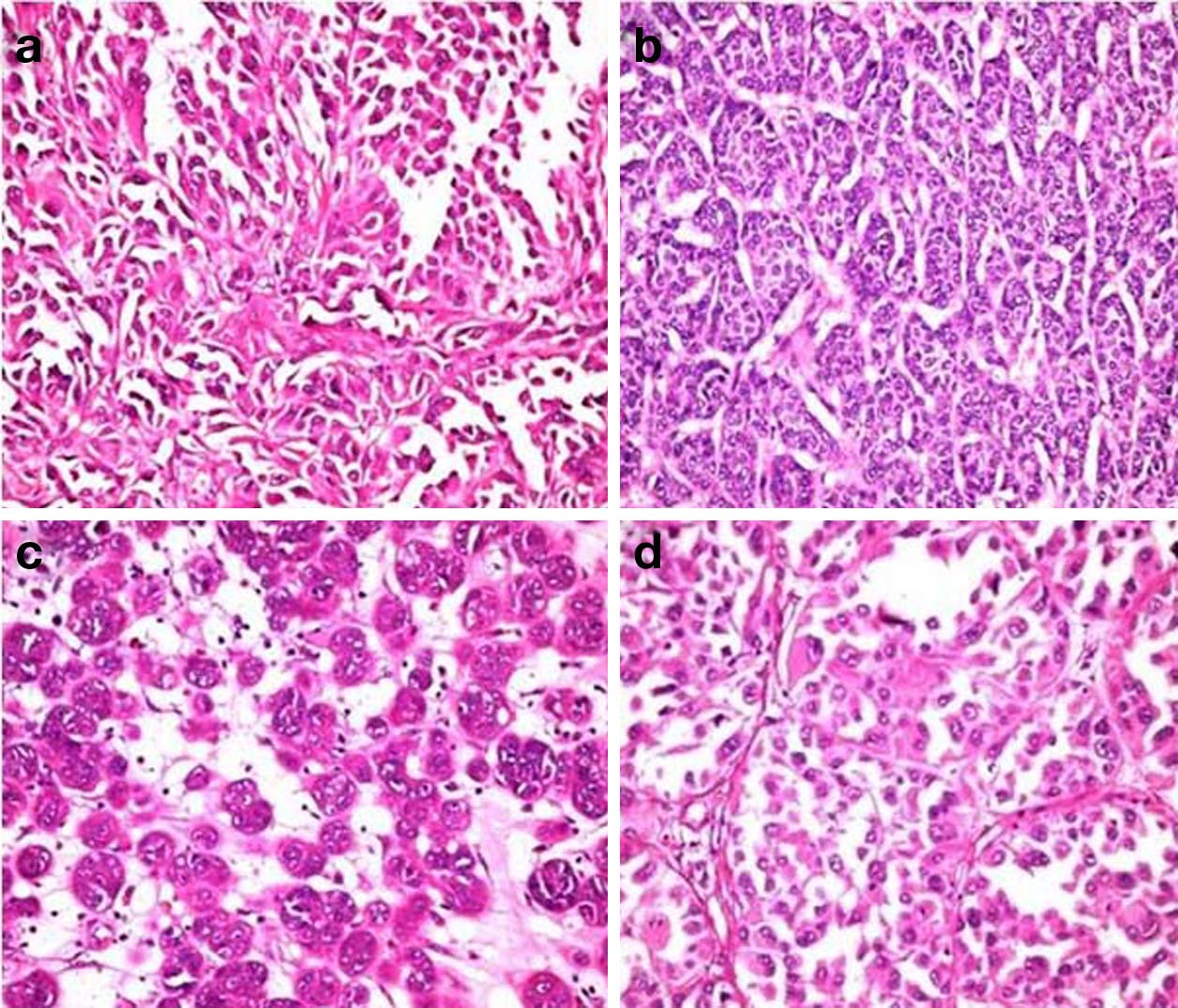
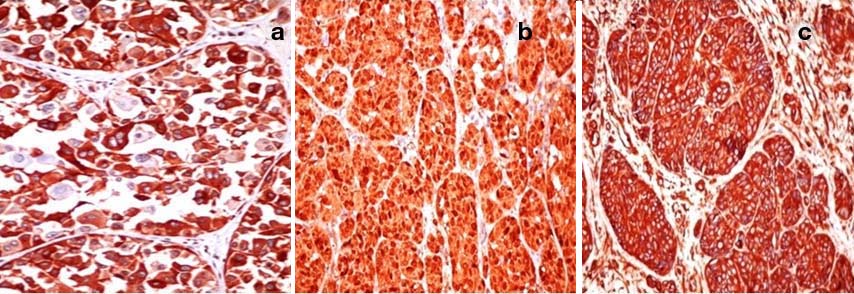
Histopathological study revealed islands, clusters and small nests of tumour cells with round to oval plump nuclei and conspicuous nucleoli arising from the basal layer of epidermis and infiltrating the dermal papillae, papillary dermis and reticular dermis with variegated morphology [Table/Fig-3a]. In few areas the tumour cells were spindle shaped resembling a sarcoma [Table/Fig-3b]. Prominent interspersed vascular channels lined by atypical cells of both spindle and epithelioid cells and foci with clear cell change were seen [Table/Fig-3c,d]. The arrangement of tumour cells varies from papillary to organoid, nesting and alveolar patterns [Table/Fig-4a-d]. There was no evidence of intracellular or extracellular melanin pigment deposition. On Immunohistochemical (IHC) study, tumour cells showed strong positivity for HMB45, S100 and vimentin [Table/Fig-5a-c]. Pancytokeratin was focal and weak positive. CD34 and CD31 were negative.
Histopathological images: a) Junctional activity with involvement of papillary, superficial and deep dermis (H&E x40); b) Short fascicles of spindle cells resembling sarcoma (H&E x400); c) Tumour cells with interspersed vascular channels resembling epithelioid variant of angiosarcoma (H&E x400); d) Cells with clear to vacuolated cytoplasm (H&E x400).
Variegated arrangement of tumour cells: a) Papillary; b) Organoid; c) Nesting; d) Alveolar (a-d H&Ex400).
Immunohistochemistry: a) HMB45 x400; b) S100 x400; c) Vimentin x400.
Based on histopathology and immunohistochemical markers, a diagnosis of amelanotic melanoma with a proliferative index (MIB1) of 4 % was made. Clark’s level was IV and TNM stage was IIC. After consideration of all the available treatment modalities, wide local excision of the tumour was done followed by skin grafting. The patient was referred to Oncology Department for further management and was on follow up. After nine months of uneventful postoperative period he suddenly developed right sided hemiparesis, persistent headache with disorientation. CT imaging scan showed a well defined cystic lesion in left frontal region with heterogenous enhancement of the solid part. Tumour tissue was resected and sent for histopathological examination which revealed metastatic amelanotic melanoma with similar morphology and immunophenotype. The patient showed symptomatic improvement after surgery and is on close follow up for the last three months without any significant complaint.
Discussion
Lymphoedema is a condition of localised fluid retention and tissue swelling caused by compromised lymphatic system. Most common cause of lymphoedema in tropical country is filariasis but in other parts of the world it is secondary to lymphatic injury following tumours, surgery and radiotherapy [1,2]. Lymphoedema following filarial infection has been associated with lymphangiosarcoma, squamous cell carcinoma, leiomyosarcoma and rarely melanoma [3–6]. Herein we report the first case of amelanotic melanoma developing in a background of chronic lymphoedema of filarial aetiology.
Risk factors for melanoma include exposure to sunlight and UV radiation, presence of dysplastic naevi, family history of melanoma, personal history of repeated sunburns, immunosuppression, including organ transplant recipients, blue or green colour eye, presence of freckles, history of penetrating injury and exposure to agricultural chemicals [7,8]. But there is no literature supporting chronic filarial oedema as a risk factor for the development of melanoma. Rekha A et al., from South India were the first to report a case of melanoma in a patient with filarial lymphoedema [5].
Majority of malignant melanomas are pigment producing. Amelanotic melanomas constitute only 2% of all melanomas [9]. The present case of concern did not show any evidence of melanogenesis either grossly or microscopically.
Survival in malignant melanoma is strongly related to tumour thickness and local and distant tissue spread at the time of diagnosis [7]. This makes early detection and diagnosis crucial, which helps in better treatment and disease free survival. Specifically in this case, diagnosis was missed and treated conservatively in line of non healing ulcer of elephantiasis. The tumour grew over a period of one and half years, attaining a size of 25 cm before a definitive diagnosis of amelanotic melanoma was made. Surprisingly there was no nodal metastasis or systemic spread at the time of diagnosis. The extensive dermal scarring of lymphatics and veins in elephantiasis might have played a role as a mechanical barrier for spread of tumour.
Edwardsiella tarda (E. tarda), a hydrogensulphide producing enterobactereriaceae, is known to cause wound infection and myonecrosis with foul smelling discharge. It is observed that, upregulation of MDA5 (Melanoma Differentiation Associated Gene 5) receptors occurs in culture cell lines following infection with E. tarda. MDA5 expression helps in release of interferon and thereby, has a protective role in viral infection [10]. Though E. tarda was isolated from the wound discharge, in the present case of concern, its role in causation of melanoma by activation and upregulation of MDA5 is yet to be established.
Obliterative fibrosis of lymphatics results in impairment of regional immune response due to decreased number of circulatory T lymphocytes which helps in eliminating tumour specific antigens. Absence of lymphatic drainage is also reported to cause accumulation of carcinogenic substances which might have played a role in inducing alternation at DNA level resulting in mutations and malignant transformation [11]. However, mutation specific for development of malignant melanoma in this clinical scenario is not yet known.
Conclusion
The oncogenic roles of several infective agents (viruses and bacteria) are well established in human cancers. We need to search for the genetic and epigenetic changes (if any) induced by filarial antigen, required for malignant transformation of melanocytes to melanoma. In the process, looking for expression of filarial antigen in tumour as well as in the non-tumourous area of filarial skin may provide some insight into the pathogenesis of the disease. All cases of non healing ulcers in filariasis need close follow up, as many times the diagnosis of malignancy is delayed or missed.
[1]. Shenoy RK, Clinical and pathological aspects of filarial lymphoedema and its managementKorean J Parasitol 2008 46:119-25. [Google Scholar]
[2]. Szuba A, Rockson SG, Lymphoedema: Classification, diagnosis and therapyVasc Med 1998 3:145-56. [Google Scholar]
[3]. Agale SV, Khan WA, Chawlani K, Chronic lymphoedema of filarial origin: A very rare aetiology of cutaneous lymphangiosarcomaIndian J Dermatol 2013 58:71-73. [Google Scholar]
[4]. Abhyankar SV, Kulkarni A, Kulkarni M, Agarwal NK, Squamous cell carcinoma of the penis and scrotum in a patient with chronic scrotal and penile lymphoedemaIndian J Dermatol 2010 55:387-89. [Google Scholar]
[5]. Rekha A, Ravi A, Venu N, Shivanraj A, Malignant melanoma and filariasis: A coexistence or an associationInt J Extrem Wounds 2005 4:60-64. [Google Scholar]
[6]. Talikoti MA, Deo SS, Shukla NK, Kallianpur AA, Gupta M, A rare case of giant leiomyosarcoma in a filarial scrotum: A case reportWorld J Surg Oncol 2011 9:20-23. [Google Scholar]
[7]. Elder DE, Elentiasis R, Murphy GF, Xu X, Benign pigmented lesions and malignant melanoma. In: Elder DE, editorLever’s Histopathology of the skin 2004 10th editionNew DelhiWolters Kluwer publishers:737-89. [Google Scholar]
[8]. Bristow IR, deBerker DA, Acland KM, Turner RJ, Bowling J, Clinical guidelines for recognition of melanoma of the foot and nail unitJ Foot Ankle Res 2010 3:25-37. [Google Scholar]
[9]. Rahbari H, Nabai H, Mehregan AH, Mehregan DA, Mehregan DR, Lipinski J, Amelanotic lentigo malignant melanomaCancer 1996 77:2052-57. [Google Scholar]
[10]. Zou PF, Chang MX, Xue NN, Liu XQ, Li JH, Fu JP, Melanoma differentiation-associated gene 5 in zebrafish provoking higher interferon-promoter activity through signalling enhancing of its shorter splicing variantImmunology 2014 141:192-202. [Google Scholar]
[11]. Turk BG, Bozkurt A, Yaman B, Ozdemir F, Unal I, Melanoma arising in chronic ulceration associated with lymphoedemaJ Wound Care 2013 22:74-75. [Google Scholar]