Renal Clear Cell Sarcoma - Anaplastic Variant: A Rare Entity
Vaishali Atmaram Walke1, Nitin Y Shende2, D T Kumbhalkar3
1 Assistant Professor, Department of Pathology, Goverment Medical College, Nagpur, Maharashtra, India.
2 Assistant Professor, Department of Pathology, Goverment Medical College, Nagpur, Maharashtra, India.
3 Professor, Department of Pathology, Goverment Medical College, Nagpur, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vaishali Atmaram Walke, 65C, Shri Gajanan Apartment, Gajanan Nagar. Wardha Road, Nagpur-440015, Maharashtra, India.
E-mail: drvaishaliw@yahoo.com
Clear Cell Sarcoma of Kidney (CCSK) is known for its morphologic diversity, aggressive behaviour, tendency to recur and metastasis to bone. Amongst the various morphologic subtypes, anaplastic CCSK is associated with worse prognosis. Here, we report a case of this rare variant of CCSK. A five-year-old boy presented with history of lump and pain in abdomen since one week. The Computed Tomography (CT) scan revealed a large mass occupying the middle and inferior pole of right kidney. The clinical impression was Wilms tumour. Nephrectomy specimen was received and the diagnosis of CCSK anaplastic variant was offered only after excluding the differentials and after performing ancillary tests such as Immunohistochemistry (IHC). Thus, this case emphasizes the diagnostic challenges on morphology and the essential role of IHC in arriving at a definitive diagnosis, because failure to do so may deprive the child from optimal treatment.
Childhood renal tumour, Paediatric kidney tumour, Sarcoma of kidney
Case Report
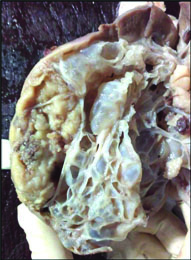
A five-year-old boy had a history of lump and pain in abdomen since one week. He was investigated for the same. His CT scan revealed a large mass occupying the middle and inferior pole of right kidney. The other organs were unremarkable. The radiological diagnosis was Wilms tumour. In view of clinico-radiological diagnosis of Wilms tumour, the surgery was planned. We received a nephrectomy specimen which was externally smooth, lobulated and congested. The cut surface revealed a huge solid-cystic tumour of size 13cm × 7cm × 5cm, occupying most of the kidney and sparing the upper pole [Table/Fig-1]. The solid area was 5.5cm ×3cm × 2.5cm.
Gross: solid-cystic tumour in centre and lower pole of size 13cm × 7cm × 5cm. Cysts are multilocular, filled with serous fluid with single solid area 5.5cm × 3cm × 2.5cm in size. Compressed normal kidney is seen in the upper pole.
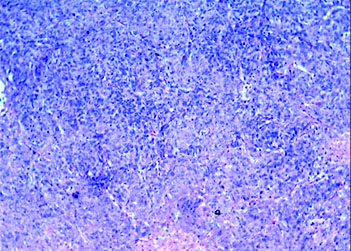
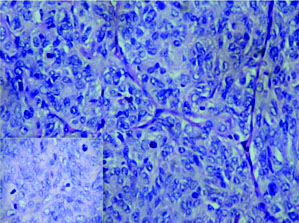
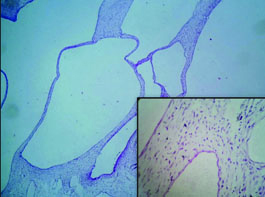
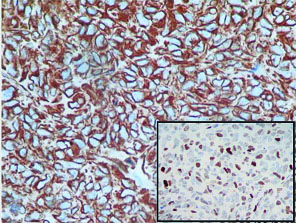
On histopathology, the tumour was well circumscribed with focal infiltration into the adjacent renal parenchyma. The cells were arranged in diffuse sheets and in an organoid fashion [Table/Fig-2]. At places fine arborizing vasculature was noted. The individual cells were oval, plump to spindle with indistinct border and scant cytoplasm. The nuclei were round to oval with fine chromatin and inconspicuous nucleoli [Table/Fig-3]. Mitotic figures including atypical mitosis and foci of coagulative necrosis were seen [Table/Fig-3 (inset)]. The cystic areas showed cysts of variable dimensions lined by low cuboidal cells and separated by thin fibrocollagenous stroma [Table/Fig-4] and [Table/Fig-4 (inset)] shows stroma at higher magnification. Putting together these features, a morphologic diagnosis of CCSK was entertained. Panel of immunomarkers was advised to exclude the closest differential of Wilms tumour. It further confirmed the anaplastic subtype. The tumour cells showed vimentin positivity [Table/Fig-5] and overexpression of p53 [Table/Fig-5 (inset)] while Cytokeratin (pan CK), EMA, Wilms tumour protein (WT1), SMA and Desmin were negative. The presence of nuclear hyperchromasia, atypical mitosis and overexpression of p53 (>75%) favoured the diagnosis of anaplastic CCSK.
The tumour cells in diffuse sheets and in organoid fashion. (H&E stain; 10X).
Tumour nests separated by thin arborizing, fibrovascular septae. The nuclear chromatin is finely granular with inconspicuous nucleoli (H&E stain 20X); Inset-atypical mitosis (H&E stain 40X). (Images left to right)
The cysts of variable dimensions lined by low cuboidal cells and separated by thin fibrocollagenous stroma (H&E stain; 10X); Inset-Stroma at higher magnification (H&E stain; 40X).
IHC-vimentin: diffuse cytoplasmic positivity (20X); Inset-p53 nuclear overexpression (10X).
Discussion
CCSK is a rare paediatric renal tumour and accounts for about 3% amongst the various renal tumours in children. CCSK was first recognized as a separate entity from Wilms tumour by Kidd in 1970 [1]. It gained importance because of its tendency to metastasize to bone and is called the bone metastasizing renal tumour of childhood [1–3]. It is often associated with tremendous morphologic diversity, poor outcome and high mortality [1]. The term clear cell sarcoma was first coined by Beckwith and Palmer due to presence of numerous intracytoplasmic vesicles [2].
The morphologic diversity and scarceness of appropriate diagnostic markers are responsible for diagnostic delay and further treatment. These are unilateral tumours arising from medullary region of kidney with necrosis and cyst formation [2]. Most of these have a classic pattern. The various other patterns are myxoid (50%), sclerosing (35%), cellular (26%), epitheloid (13%), spindle (7%), storiform (4%) and anaplastic (2.6%) [1]. It has already been conceptualized that the various patterns of CCSK can best be understood as alterations of either cord or septal cells morphology [1]. In the classic type, the cells are polygonal to round in solid nests or cords separated by delicate, arborizing fibrovascular septa. The nuclei have evenly dispersed chromatin and inconspicuous nucleoli. The cells have clear to pale eosinophilic cytoplasm [1–3]. The variant histopathologic patterns identifies no prognostic correlation, however they do highlight differential diagnostic dilemmas. Of particular interest is the distinction of CCSK from blastemal Wilms tumour having prominent vascular pattern. In blastemal predominant Wilms tumour, the cells are compactly arranged with nuclei in close approximation with overlapping nuclei, coarse chromatin and little intervening cytoplasm. While the nuclei in CCSK are widely spaced having fine chromatin pattern [4]. A careful search reveals epithelial differentiation in Wilms tumour in the other areas of tumour.
An equally challenging diagnostic problem is the distinction of epitheloid CCSK from epithelial predominant Wilms tumour. The search for blastemal component in Wilms tumour along with classical arborizing vascular pattern of CCSK will help us to differentiate between these two conditions [5]. Wilms tumour is aggressively invasive than CCSK, entrapping whole islands of renal parenchyma as opposed to the single tubule entrapped by CCSK [3]. At times these entrapped tubules get dilated extensively giving rise to grossly evident cysts with pauci cellular septae [1]. In rare cases exaggerated cystic appearance mimics with cystic nephroma on microscopy [1]. Predominant cystic component was also seen in our case. IHC plays an important role to make this distinction possible in case of doubt. None of the variants of CCSK show cytokeratin expression. So also WT1 positivity supports the diagnosis of Wilms tumour over CCSK. Vimentin is consistently and uniformly expressed in CCSK [1–4]. In the present case vimentin showed diffuse cytoplasmic positivity while cytokeratin and EMA were negative in the tumour cells; however it has highlighted the entrapped cystically dilated tubules in the peripheral part of the tumour [2]. The expression of WT1 was absent which favoured the diagnosis of CCSK over Wilms tumour in our case. Spindle cell variant of CCSK is difficult to distinguish from plump cell variant of cellular mesoblastic nephroma. These patients of mesoblastic nephroma generally present within first three months of life and show short interlacing fascicles of spindle cells. Their nuclei have coarse chromatin and prominent nucleoli [4,6]. Besides its fine chromatin, the best clue to CCSK is the presence of its classic pattern which may be absent in small biopsy specimens. In our case, both desmin and S100 were negative; positivity for either of these markers can be used as strong evidence against CCSK [4]. The main contribution of IHC is to exclude other diagnostic possibilities.
The anaplastic variant of CCSK is characterized by nuclear hyperchromasia, nuclear gigantism and atypical mitosis with overexpression of p53 in the anaplastic foci [1]. The presence of nuclear hyperchromasia, atypical mitosis and overexpression of p53 in the anaplastic area supported the diagnosis of anaplastic subtype of clear cell sarcoma in the present case. The unusual finding in our case was the membranous positivity of CD99 which is a rare phenomenon [6–8]. Management for CCSK at all stages require aggressive surgical approach followed by chemotherapy and radiotherapy as per NWTS-5 protocols [9,10]. The four important prognostic factors are treatment with doxorubicin, stage, age at diagnosis and tumour necrosis [9]. The essential role of addition of doxorubicin in therapy emphasizes the need for pathologist to accurately identify CCSK. Failure to recognize the tumour as CCSK could deprive the child of optimal chemotherapy [9,10].
Conclusion
To conclude CCSK is a frequently misdiagnosed tumor due to tremendous morphologic diversity where in the primary contribution of IHC is to exclude other diagnostic possibilities. The accurate and timely diagnosis is also critical because of its aggressive behaviour.
Abbreviations
H&E–Haematoxylin and Eosin
CK–Cytokeratin
IHC–Immunohistochemistry
CCSK–Clear cell sarcoma of Kidney
NWTS–National Wilms Tumour Study
CD–Cluster Differentiation
[1]. Argani P, Perlman EJ, Breslow NE, Browning NG, Green DM, D’Angio GJ, Clear cell sarcoma of the kidney: A review of 351 cases from the National Wilms Tumour Study Group Pathology CentreThe Am J of Surg Pathol 2000 24:4-18. [Google Scholar]
[2]. Gooskens SLM, Furtwangler R, Vujanic GM, Dome JS, Graf N, van den Heuvel-Eibrink MM, Clear cell sarcoma of the kidney: A reviewEur J of Cancer 2012 28:2219-26. [Google Scholar]
[3]. Lal N, Singhai A, Clear cell sarcoma of kidney: A rare entityIndian J of Med Pediatr Oncol 2011 32:157-59. [Google Scholar]
[4]. Argani P, Beckwith JB, Renal neoplasms of childhood. In: Mills SE, Carter D, Greenson JK, Reuter VE, Stoler MH. (editors)Sternberg’s Diagnostic Surgical Pathology 2010 vol II5th edNew YorkWolters Kluwer, Lippincott Williams & Wilkins:1815-19. [Google Scholar]
[5]. Biswas PK, Mandal PK, Nag D, Nandi A, Clear cell sarcoma of the kidney: A case reportJ of Cancer Research and Therapeutics 2014 10:1104-06. [Google Scholar]
[6]. Alavi S, Khoddami M, Yazdi MK, Dehghanian P, Esteghamati S, Clear cell sarcoma of kidney misdiagnosed as mesoblastic nephroma: A case report and review of literatureE Cancer Medical Science 2013 7:311 [Google Scholar]
[7]. Short S, Zmora O, Hunter CJ, Wang L, Siegel S, Ford HR, Large clear cell sarcoma of the kidney mistaken as Wilms tumourJ Ped Surg Case Reports 2013 1:235-38. [Google Scholar]
[8]. Hirose M, Mizuno K, Kamisawa H, Nishio H, Moritoki Y, Kohri K, Clear cell sarcoma kidney distinguished from synovial sarcoma using genetic analysis: A case reportBMC Research Notes 2015 8:129 [Google Scholar]
[9]. Roy H, Mondal P, Dey S, Roy S, Clear cell sarcoma of kidney - A report of two casesAl Ameen J Med Sci 2011 4:401-04. [Google Scholar]
[10]. El-Hawari AK, Zalata KR, Abd Elrehim M, Abdel Hamid M, El-Hefnawi AS, Abou Bieh EA, Adult clear cell sarcoma of the kidney: A case reportOA Case Reports 2013 21:111 [Google Scholar]