Invasive Papillary Carcinoma of the Male Breast Misdiagnosed as Fibroadenoma on FNAB
Richa Katiyar1, Shashikant C.U. Patne2, Sandip Kumar3, Rahul Khanna4
1 Service Senior Resident, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
2 Assistant Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
3 Assistant Professor, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
4 Professor, Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shashikant C.U. Patne, Department of Pathology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India.
E-mail: scup.pathology08@gmail.com
Male breast cancers constitute less than 1% of all the breast cancers. Papillary carcinoma is a very rare tumour of the male breast. Due to rarity, Fine Needle Aspiration Biopsy (FNAB) findings of papillary carcinoma in male breast are seldom reported. A 55-year-old male presented with a lump in the left breast of two years’ duration. FNAB was reported as fibroadenoma. Histopathological examination of the excised breast lump revealed invasive papillary carcinoma. Immunohistochemistry showed expression of pancytokeratin, oestrogen receptor, and progesterone receptor. Negative immunostaining was seen for HER2, p53, 34βE12, and CD34. Ki-67 proliferative index was 5%. We have discussed cytological findings of invasive papillary carcinoma and its differential diagnoses. Cytopathologists must be aware of cytologic findings of invasive papillary carcinoma of the male breast.
Cytology, Immunohistochemistry, Hormone receptors, Malignant
Case Report
A 55-year-old male presented with two years’ history of progressive painless enlargement of the left breast lump. His family history for breast cancer was negative. His left breast retro-areolar lump measuring 6×4 cm was firm, mobile, and non-tender. The right breast was unremarkable. In some other hospital, FNAB was done from his left breast lump and was reported as fibroadenoma. FNAB slides showed moderately cellular smears comprised of papillaroid clusters and branching cohesive sheets of ductal cells exhibiting uniform round nuclei and scattered bare bipolar nuclei. Further, occasional clusters of ductal cells showed columnar arrangement of nuclei with nuclear overlapping and mild hyperchromasia [Table/Fig-1a]. Few scattered ductal cells and sparse bipolar nuclei were noted around the latter clusters. Masood Cytologic Index (MCI) was applied on FNAB smears that showed following scores: cellular arrangement-4; cellular pleomorphism-2; myoepithelial cells-3; anisonucleosis-2; nucleoli-1; and chromatin clumping-1. The total MCI score obtained was 13, which was categorized as proliferative breast disease without atypia [1]. The patient’s routine preoperative laboratory investigations were within normal limits.
(a) FNAB smear showing overlapping clusters of ductal cells with few dyscohesive cells in the background admixed with bare bipolar nuclei (Pap stain, 400X); (b) Cut surface showing solid grey white infiltrative tumour in the breast parenchyma; (c) Well-defined papillary tumour with fibrovascular core (H&E stain, 100X).
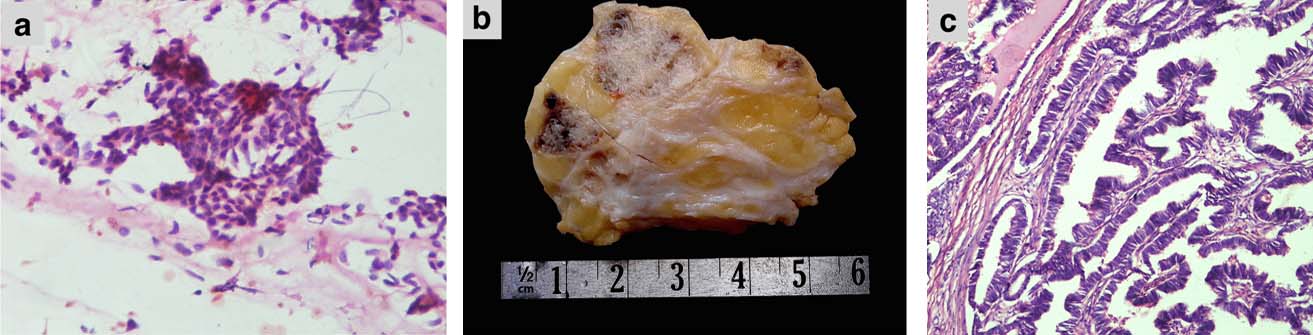
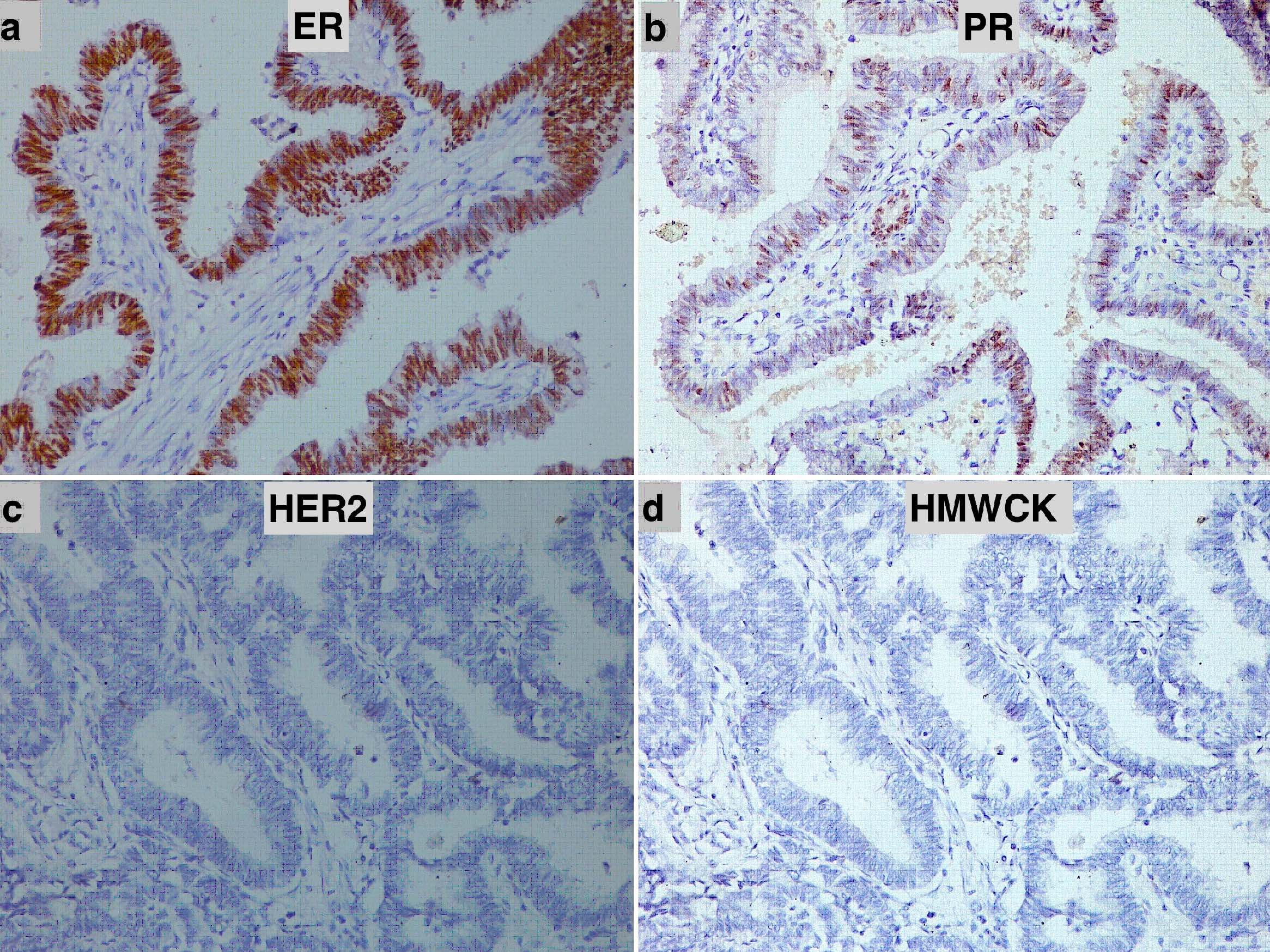
He underwent left breast simple mastectomy that was submitted for histopathological examination. Grossly, the specimen with nipple and areola measured 6×4×3 cm. Cut sections showed a solid grey-white tumour of size 3×2×1.5 cm in the central quadrant [Table/Fig-1b]. Microscopic examination showed breast parenchyma infiltrated by a solid-cystic papillary tumour lined by tall columnar epithelium having hyperchromatic nuclei, inconspicuous nucleoli, and 8-10 mitoses/10 high power fields [Table/Fig-1c]. The peripheral part of the tumour showed foci of cribriform type of ductal carcinoma in situ. Cyst lumen showed secretions and occasional microcalcifications. Lymphovascular or perineural invasion were not seen. Immunohistochemistry on paraffin blocks with standard procedure revealed immunoreactivity of the tumour for pancytokeratin, oestrogen receptor (Allred score 8) [Table/Fig-2a] and progesterone receptor (Allred score 5) [Table/Fig-2b]. The tumour cells were immunonegative for HER2 [Table/Fig-2c], high molecular weight cytokeratin-34βE12 [Table/Fig-2d], p53, and CD34. Ki-67 proliferative index was 5%. The final histopathological diagnosis was invasive papillary carcinoma of the male breast. A 6-month follow up did not show any evidence of recurrence or metastasis.
Immunohistochemical staining in male breast papillary carcinoma: (a) Oestrogen receptor (IHC, 200X); (b) Progesterone receptor (IHC, 200X); (c) HER2 (IHC, 200X); and (d) high molecular weight cytokeratin-34βE12 (IHC, 200X).
Discussion
Male breast cancers constitute less than 1% of all the breast cancers. The most common histological type of the male breast cancer is infiltrating ductal carcinoma [2]. Papillary carcinoma of the male breast is a very rare tumour, accounting for less than 5% of all male breast cancers [2]. Due to rare occurrence, FNAB findings of invasive papillary carcinoma of the male breast are seldom reported [3–9].
As such, papillary lesions of the breast are diagnostically challenging for cytopathologists due to overlapping features with diverse benign and malignant diagnoses [10,11]. Cytological criteria for diagnosing papillary lesions include richly cellular smears showing branching epithelial sheets, three dimensional epithelial cell groups with or without true papillary fragments, scattered epithelial cells, mild to moderate nuclear atypia, palisaded columnar epithelial cells, apocrine metaplasia, macrophages, mitoses, and variable amount of bare bipolar nuclei in a haemorrhagic background [11].
On aspiration biopsy, often it is difficult to distinguish between true papillary lesions and lesions having a papillary component like fibroadenoma, fibrocystic disease, papillary type Ductal Carcinoma In Situ (DCIS), and infiltrating ductal carcinoma with papillary structures. Among these, fibroadenoma and fibrocystic disease of the breast closely mimic papillary lesion of the breast on FNAB [12]. We experienced a similar problem in this case, where lack of cellular clusters with fibrovascular cores and absence of overt cytological features of malignancy led to misdiagnosis of fibroadenoma. Cytologic smears of fibroadenoma are usually cellular with large branching sheets of ductal epithelial cells, fibromyxoid stromal fragments with many bare bipolar nuclei in the background. In comparison to fibroadenoma, the smears of this case contained neither abundant bare bipolar nuclei nor fibromyxoid stromal fragments. The absence of these two cytological features of fibroadenoma along with occasional overlapping clusters of ductal cells showing columnar-palisaded nuclei and mild nuclear atypia should raise a suspicion for invasive papillary carcinoma of the male breast. The FNAB smears of fibrocystic disease are less cellular with fragments of epithelial cells, apocrine metaplasia, and background showing macrophages, cyst fluid and dispersed bare bipolar nuclei. Cytology smears from papillary type DCIS reveal sheets of ductal epithelial cells with mild-to-moderate nuclear atypia, some nuclear crowding, papillary fragments, and absence of bare bipolar nuclei [10]. Background may show necrosis. Smears from infiltrative ductal carcinoma with focal papillary components are highly cellular with scattered and clusters of neoplastic epithelial cells showing moderate to marked nuclear atypia, nuclear crowding, clumped chromatin, irregular nuclear membrane and absence of bare bipolar nuclei in background [10]. Micropapillary carcinoma of the breast is a rare entity, which lacks true fibrovascular core. Overall, FNAB smears from malignant papillary lesions are highly cellular with presence of branching complex papillae, dispersed neoplastic epithelial cells showing moderate nuclear atypia and absence of bare bipolar nuclei and macrophages. Differentiation between invasive and non-invasive papillary carcinoma is difficult as both show similar cytological features [10].
In papillary lesions of the breast, demonstration of loss of myoepithelial cell layer by immunohistochemistry is taken as the evidence of malignancy. Loss of high molecular weight cytokeratins (CK5, CK5/6, CK14, and 34βE12) and/or myoepithelial markers (p63 and smooth muscle actin) establishes loss of myoepithelial cell layer and confirms the diagnosis of papillary carcinoma of the breast [13]. We have demonstrated loss of 34βE12 around the papillary clusters in our case. Recently, loss of CD133 has been noted in papillary carcinoma of the breast [14]. Distinction of papillary carcinoma from benign papillary lesions of the breast is also facilitated by genetic features. Loss of heterozygosity of chromosome16q23 was found to be specific for papillary carcinoma. Alterations in chromosomes 3, 7, 17 and X by multicolour fluorescent in situ hybridization have also been shown in all cases of DNA-aneuploid carcinoma. Thus, study of DNA-ploidy may also be useful in the diagnosis of papillary carcinoma [15]. The prognostic and predictive factors for the male and the female breast carcinomas are similar.
Conclusion
In conclusion, cytopathologists must be aware of rare diagnosis of invasive papillary carcinoma involving the male breast. Although FNAB is an easy and cost-effective procedure in the diagnosis of breast lesions, its careful interpretation is required in male breast lumps. Accurate preoperative diagnosis may be difficult. Histopathological and immunohistochemical examinations are mandatory to arrive at correct diagnosis in such cases.
[1]. Rekha T, Nandini N, Dhar M, Expansion of Masood’s cytologic index for breast carcinoma and its validityJ Cytol 2013 30:233-36. [Google Scholar]
[2]. Burga AM, Fadare O, Lininger RA, Tavassoli FA, Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 casesVirchows Arch 2006 449:507-12. [Google Scholar]
[3]. Khalbuss WE, Ambaye A, Goodison S, Loya A, Masood S, Papillary carcinoma of the breast in a male patient with a treated prostatic carcinoma diagnosed by fine-needle aspiration biopsy: a case report and review of the literatureDiagn Cytopathol 2006 34:214-17. [Google Scholar]
[4]. Romanelli R, Toncini C, Pigmented papillary carcinoma of the male breastTumouri 1986 72:105-08. [Google Scholar]
[5]. Kumar PV, Talei AR, Malekhusseini SA, Monabati A, Vasei M, Papillary carcinoma of the breast. Cytologic study of nine casesActa Cytol 1999 43:767-70. [Google Scholar]
[6]. Joshi N, Pande C, Papillary carcinoma of the male breast diagnosed by fine needle aspiration cytologyIndian J Pathol Microbiol 1998 41:103-06. [Google Scholar]
[7]. Mockli G, Hanks D, Jeffrey PB, Papillary carcinoma of the male breast. Report of a case diagnosed by fine needle aspiration cytologyActa Cytol 1993 37:721-24. [Google Scholar]
[8]. Arora R, Gupta R, Sharma A, Dinda AK, Invasive papillary carcinoma of male breastIndian J Pathol Microbiol 2010 53:135-37. [Google Scholar]
[9]. Pant I, Joshi SC, Invasive papillary carcinoma of the male breast: report of a rare case and review of the literatureJ Cancer Res Ther 2009 5:216-18. [Google Scholar]
[10]. Aggarwal D, Soin N, Kalita D, Pant L, Kudesia M, Singh S, Cytodiagnosis of papillary carcinoma of the breast: Report of a case with histological correlationJ Cytol 2014 31:119-21. [Google Scholar]
[11]. Jeffrey PB, Ljung BM, Benign and malignant papillary lesions of the breast. A cytomorphologic studyAm J Clin Pathol 1994 101:500-07. [Google Scholar]
[12]. Simsir A, Waisman J, Thorner K, Cangiarella J, Mammary lesions diagnosed as “papillary” by aspiration biopsy: 70 cases with follow upCancer 2003 99:156-65. [Google Scholar]
[13]. Tse GM, Ni Y-B, Tsang JYS, Shao M-M, Huang Y-H, Luo M-H, Immunohistochemistry in the diagnosis of papillary lesions of the breastHistopathology 2014 65:839-53. [Google Scholar]
[14]. Lin C-H, Liu C-H, Wen C-H, Ko P-L, Chai C-Y, Differential CD133 expression distinguishes malignant from benign papillary lesions of the breastVirchows Arch 2015 466:177-84. [Google Scholar]
[15]. Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, Papillary carcinoma of the breast: an overviewBreast Cancer Res Treat 2010 122:637-45. [Google Scholar]