Treatment of periodontitis involves non-surgical periodontal therapy aimed at eliminating the aetiological factors like bacterial flora responsible for inflammation, but has a shortcoming as it only limits the progression of disease where as surgical therapy, is directed at resolving the defects and has the capacity to enhance periodontal regeneration [1,2]. Periodontal regeneration refers to the restoration of supporting tissues of the teeth, which are the alveolar bone, cementum and periodontal ligament to their original healthy levels, before the tissue destruction occurred [3].
Various modalities that enhance periodontal regeneration include numerous graft materials derived from both natural and synthetic sources and Guided Tissue Regeneration (GTR). An alloplast is a synthetic graft or inert foreign body implanted into the tissues. Bioceramic alloplasts have a composition similar to that of bone comprising primarily of calcium phosphate, with proportion of calcium and phosphate. The two most widely used forms of bioceramic alloplasts are tricalcium phosphate and hydroxyapatite [4]. A porous form of calcium phosphate viz., β-TCP is the most commonly used alloplastic periodontal regenerative material. It serves as biological filler which is partially resorbable and allows bone replacement [5].
Many management options are available in the armory of a periodontist including surgical as well as non-surgical therapy, regenerative techniques and the most latest tissue engineering technology has also been included [6]. Not satisfied with the traditional weapons the researchers have turned towards novel pharmacological molecules and bone sparing agents to combat periodontal disease [6]. Pharmacological agents like alendronate, simvastatin, teriparatide and Cyclosporine A (CsA) are extensively used in general medicine for varied indications. They have also been found to influence periodontal tissue healing and improved alveolar bone repair/regeneration [7].
One of such novel drugs viz., CsA, an immunosuppressant, considered a life saver drug in organ transplant cases has been tested in animal and human studies for periodontal applications [8]. CsA selectively inhibits T lymphocyte proliferation, IL-2 and other cytokine production, without any effect on T suppressor cells. Hence, it suppresses cell mediated immunity suppressing the inflammation [9]. Inflammatory and immunological responses have been found to be decreased and bone formation is found to be increased in immunosuppressed animals. CsA has the potential to enhance osseous regeneration due to it’s ability to increase bone alkaline phosphatase levels and a direct activating effect on the bone forming cells that is the osteoblasts [8,10].
Taking into account the effects of the drug and the available evidence of success of local application of CsA in animal studies and a lone human study, [11–15] an attempt was made In the present study to evaluate the efficacy of CsA (2 mg) as an adjunct to β-TCP for regenerative treatment of periodontal infrabony defects.
Materials and Methods
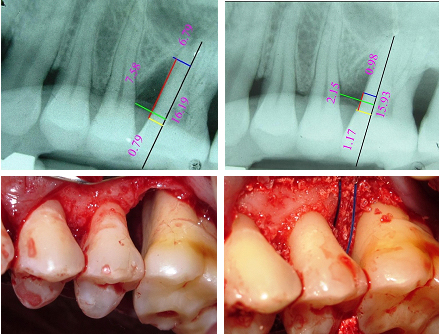
The present study was designed as a randomized, controlled, parallel arm, pilot study, evaluating the use of CsA with β-TCP or β-TCP alone In the management of periodontal infrabony defects. The study was conducted from December 2012 to March 2014. Ethical clearance (Reg no.225/sscds/IRB-E/2011) was obtained from the Institutional Ethics Committee of Sri Sai College of Dental Surgery, Vikarabad, Telangana, India. The study design comprised of eight visits, two during pre-implantation, one during implantation and five visits during post-lmplantatlon phase [Table/Fig-1]. Neither the patient nor the investigator who assessed the outcomes was aware of the group assignment, thereby assuring double-blindness.
Population Screening
A total of 41 patients were assessed for the eligibility, of which 37 patients were selected based on the inclusion and exclusion criteria.
Patient Criteria
Systemically healthy patients with chronic periodontitis, age ranging from 18-60 years, with at least one tooth having PD and CAL ≥5 mm associated with radiographic evidence of angular bone loss and an infrabony defect of ≥3 mm, were included in the study [16]. Vital teeth or teeth treated with root canal therapy were considered. Patients with good oral hygiene, presenting with Full- Mouth Plaque Scores (FMPS) and Full-Mouth Bleeding Scores (FMBS) ≤20%, were included. immunocompromised patients, patients with known allergy to immunosuppressants, patients on usage of drugs like calcium channel blockers, antifungal agents, glucocorticoids and HIV protease inhibitors like indinavir were excluded. Pregnant or lactating mothers, smokers and patients with a history of periodontal surgery in respect to the study tooth within one year, were excluded from the study. Teeth with mobility greater than grade II [17] and furcation involvement (class II and class III) [18] or with clinical signs of untreated acute infection at surgical site, were excluded.
After the Cause Related Treatment (CRT), patients were explained about the study and written informed consent was obtained. Five patients were excluded as they were reluctant to participate in the study. Thus 32 (16 male and 16 female) patients were randomly assigned into Group A and Group B by computer generated block randomization.
Implant Material
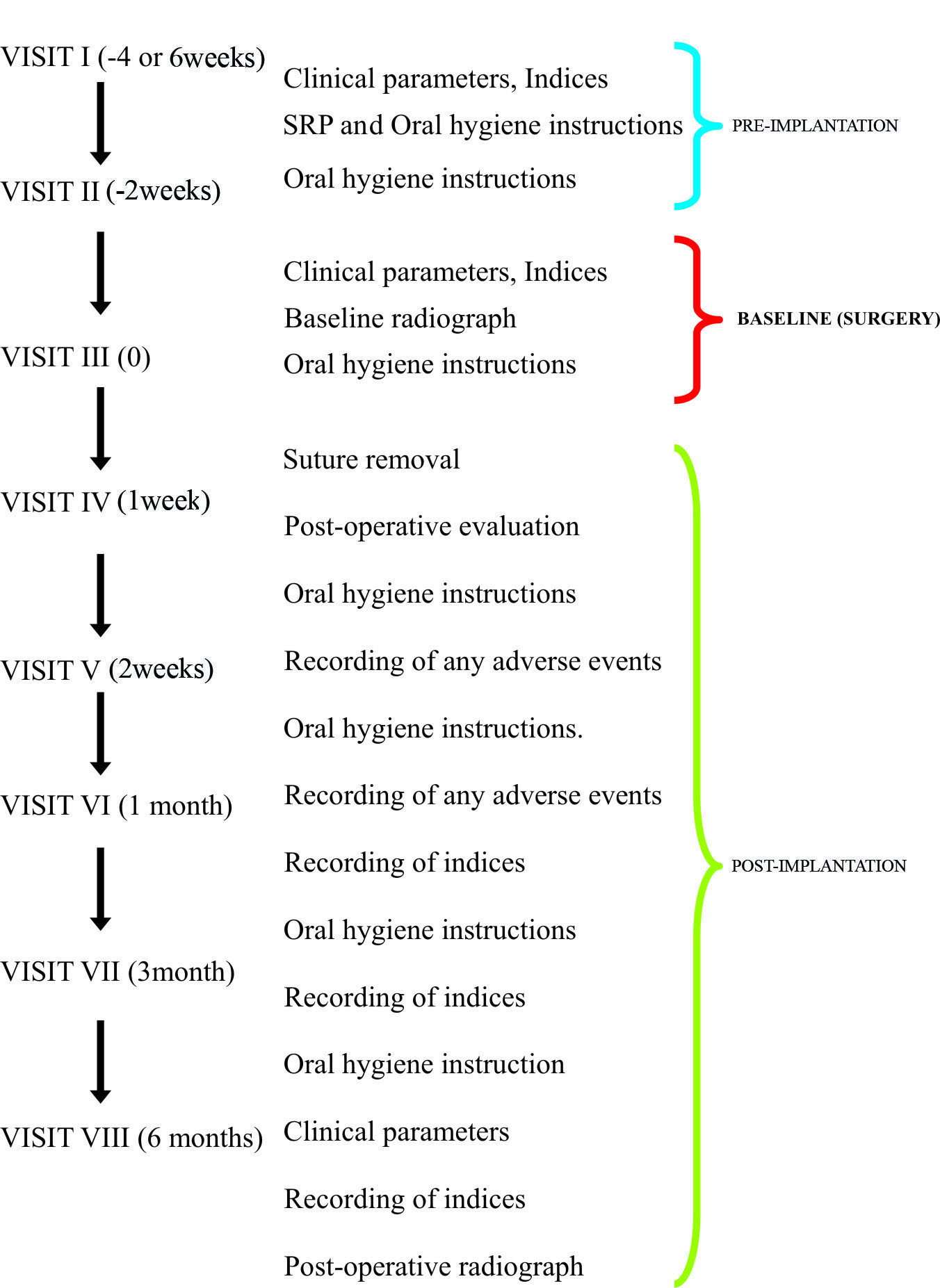
In Group A, β–TCP was mixed with CsA powder (2 mg), and in Group B, β–TCP alone was placed locally in the infrabony defects. The clinical and radiographic images have been shown in [Table/ Fig-2a-d, 3a-d].
a) Radiographic parameters at baseline; b) Radiographic parameters at six months post surgery; c) Reflection and debridement; d) Pre-suturing and graft placement.
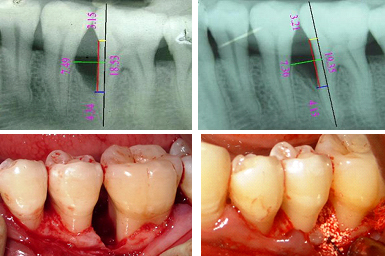
a) Radiographic parameters at baseline; b) Radiographic parameters at six months post surgery; c) Reflection and debridement; d) Presuturing and graft placement.
Clinical Parameters
The clinical parameters PD, Relative Attachment Level (RAL) and Gingival Recession (GR) were assessed at baseline and 6 months post-operatively. FMPS [19], FMBS and Modified Gingival Index (MGI) [20] at baseline, one month, three months and six months post-operatively were recorded to the nearest millimeter with the help of a UNC-15 probe on a prefabricated occlusal stent [21]. The clinical outcome parameters, Clinical Attachment Level Gain (CAL-G), Probing Depth Reduction (PD-R) and Change in Gingival Recession (CGR) were assessed at six months post-operatively.
Radiographic Parameters
Intra-oral Periapical Radiographs (IOPAR) of the selected teeth were taken using long cone paralleling technique at baseline and six months post-surgery [Table/Fig.-2a,b, 3a,b]. The Rinn XCP film holding paralleling device was used to position the films throughout the study. The radiographs were digitized and analyzed using AutoCAD software.
The anatomical landmarks of the intrabony defect were selected on the radiographs based on the criteria set by Schei O et al., [22], which included Cemento Enamel Junction (CEJ), Alveolar Crest (AC) and Base of the Defect (BD). An auxiliary line was drawn in the direction of tooth axis (AUX1) and a secondary auxiliary line (AUX2) was drawn from AC, perpendicular to the AUX1 line. The distance from the point where AUX2 crossed the CEJ-BD line to the base of the defect was measured as defect depth. Radiographic assessment was made to assess the outcome parameters, Linear Bone Growth (LBG), %BF and Change in Alveolar Crest Position (C-ACP) [23].
Surgical Procedure
After a pre-surgical rinse, the surgical area was swabbed with antiseptic and the operative site was anesthetized with 2% xylocaine hydrochloride with 1:100000 adrenaline. Full thickness mucoperiosteal flaps were reflected using the periosteal elevator. A thorough surgical debridement of the osseous defect was performed using Gracey curettes. Once the debridement was complete, the vertical defect depth (from the bottom of the defect to the alveolar crest), was recorded. Then the defect site was scraped with a periosteal elevator for the procurement of patient’s blood, which was mixed with implant material. In Group A, [Table/Fig-2c,d] β-TCP with CsA (2 mg) powder was placed in the defects, unlike group B [Table/Fig-3c,d] where β-TCP alone was placed. Prior to the graft placement pre-suturing was done with 4-0 prolene suture. The presutured mucoperiosteal flaps were repositioned and secured with interrupted sutures. The surgical area was protected and covered with non-eugenol (Coe pack) periodontal dressing. Post-operative antibiotics, viz., amoxicillin 500 mg thrice daily for five days, and analgesics ibuprofen (400 mg) and paracetemol (500 mg), eight hourly as needed were prescribed for all the patients. Appropriate postoperative instructions were given to the patients. Patients were recalled after one week for the removal of periodontal dressing and sutures. Symptoms regarding discomfort, pain, swelling, and fever were asked and recorded.
Statistical Analysis
Statistical analysis was done using SPSS version 16 software. A p-value of <0.05 was considered statistically significant. Intra group analysis of FMPS, FMBS, MGI, PD, RAL, GR, CEJ-BD, DD and CEJ-AC using Student’s paired t-test and inter-group analysis of CAL-G, PD-R, CGR, LBG, C-ACP, %BF was done using Student’s independent t-test.
Results
Each patient was considered as a unit for evaluation of results. Out of 32 patients, 27 patients completed the six months follow up. Three patients from Group A and two patients from the Group B, were lost to follow up. Three of them migrated to distant places and two were unwilling to come for the postoperative follow up visits (consort diagram [Table/Fig-4]. In all the patients, healing was uneventful and there were no systemic or local complications and adverse effects. Both the groups were evenly matched at the beginning of the study with reference to clinical and radiographic parameters as well as demographic details [Table/Fig-5]. Except for indices, which were measured at baseline, three months and six months, all other clinical and radiographic parameters were measured at baseline and six months.
Consort diagram showing study design.
Comparison of baseline, clinical and radiographic defect characteristics between both the groups.
| Baseline parameters | Group A (N=13) | Group B (N= 14) | p-value |
|---|
| Mean age | 32.06 | 29.68 | |
| PD (mm) | 7.31±1.18 | 7.64±1.45 | 0.518 (NS) |
| RAL (mm) | 10.85±1.57 | 11.57±1.83 | 0.281 (NS) |
| GR (mm) | 0.38±0.87 | 0.50±0.65 | 0.698 (NS) |
| FMPS % | 16.81±3.61 | 16.91±3.48 | 0.938 (NS) |
| FMBS % | 16.98±2.73 | 17.61±2.32 | 0.528 (NS) |
| MGI | 0.28±0.11 | 0.28±0.09 | 0.975 (NS) |
| Defect depth (mm) | 3.68±0.93 | 3.68±0.93 | 0.998 (NS) |
| Defect location: Number in maxilla Number in mandible | 10 | 6 | |
| 6 | 10 | |
| Defect associated tooth multi-rooted teeth Single-rooted teeth | 12 | 13 | |
| 4 | 3 | |
S: Statistically significant (p ≤0.05)*, NS: No statistical significance (p >0.05)
Clinical Parameters
The outcome parameters (CAL-G, PD-R, and CGR) were calculated and compared for both the groups. For Group A, mean PD, RAL and GR were 7.31±1.18 mm, 10.85±1.57 mm, 0.38±0.87 mm and at six months postoperatively were 4.69±1.25 mm, 8.54±2.07 mm, 0.62±1.04 mm respectively. For Group B, mean PD, RAL and GR were 7.64±1.45 mm, 11.57±1.83 mm, 0.50±0.65 mm and at six months postoperatively were 4.71±1.73 mm, 9.00±2.11 mm, 0.86±0.86 mm respectively. Intra-group comparisons of mean PD and RAL from baseline to six months post-operatively was statistically significant for both the test (p<0.001) and control groups (p<0.001). Intra-group comparison of mean GR from baseline to six months postoperatively was not statistically significant for both test (p=0.082) and control groups (p=0.055) [Table/Fig-6].
Intra-group analysis of primary clinical and radiographic parameters for both the groups using the student’s paired t test.
| Baseline | six months post-operative | p-value |
|---|
| Mean±SD | Mean±SD |
|---|
| Group | A | PD(mm) | 7.31±1.18 | 4.69±1.25 | < 0.001; (S)* |
| RAL(mm) | 10.85±1.57 | 8.54±2.07 | <0.001; (S)* |
| GR(mm) | 0.38±0.87 | 0.62±1.04 | 0.082 (NS) |
| CEJ-BD(mm) | 5.46±1.12 | 3.56±1.22 | 0.001 (S)* |
| DD(mm) | 3.68±0.93 | 1.61±0.91 | <0.001 (S)* |
| CEJ-AC(mm) | 1.78±1.00 | 1.95±1.05 | 0.196 (NS) |
| B | PD(mm) | 7.64±1.45 | 4.71±1.73 | <0.001; (S)* |
| RAL(mm) | 11.57±1.83 | 9.00±2.11 | <0.001; (S)* |
| GR(mm) | 0.50±0.65 | 0.86±0.86 | 0.055 (NS)* |
| CEJ-BD(mm) | 5.78±1.32 | 3.75±1.93 | <0.001 (S)* |
| DD(mm) | 3.68±0.66 | 1.62±1.04 | <0.001 (S)* |
| CEJ-AC(mm) | 2.10±1.21 | 2.05±1.17 | 0.707 (NS)* |
S: Statistically significant (p≤0.05)*, NS: No statistical significance (p>0.05)
The mean values for PD-R, CAL-G and CGR were 2.62±1.05 mm, 2.38±1.12 mm, -0.23±0.44 mm respectively for Group A and 2.93±1.27 mm, 2.57±1.22 mm, -0.36±0.63 mm respectively for Group B. Inter-group comparison for PD-R, CAL-G and CGR revealed no statistical significance for Group A as compared to the Group B (p> 0.05) [Table/Fig-7]. Intra-group comparisons of mean FMPS, FMBS and MGI at baseline, three months and six months showed statistically significant difference for both the groups (p<0.001). Inter-group comparison showed no statistical significance of neither FMPS, FMBS nor MGI (p>0.05).
Inter-group analysis of primary clinical and radiodgraphic parameters between both the groups.
| Outcome parameters | Group A | Group B | p-value |
|---|
| CAL-G | 2.38±1.12mm | 2.57±1.22mm | 0.082 (NS) |
| PD-R | 2.62±1.05mm | 2.93±1.27mm, | 0.196 (NS) |
| CGR | −0.23±0.44mm | −0.36±0.63mm | 0.707 (NS) |
| LBG | 1.90±1.48mm | 2.03±1.16 mm | 0.798 (NS) |
| %BF | 49.83±29.23% | 62.84±29.70 % | 0.156 (NS) |
| C-ACP | −0.17±0.43mm | −0.05±0.56 mm | 0.698 (NS) |
S: Statistically significant (p≤0.05)*, NS: No statistical significance (p>0.05)
Radiographic Parameters
Final outcome parameters (LBG, %BF, C-ACP) were calculated from the primary radiographic parameters for both the groups. Intra group comparison of mean CEJ-BD, DD from baseline to six months post-operatively for both the groups was statistically significant (p<0.001), whereas, intra group comparison of CEJ-AC from baseline to six months post-operatively for both the groups revealed no statistical significance with p-value, 0.196 (Group A) and 0.707 (Group B) [Table/Fig-6].
LBG, % BF, and C-ACP were 1.90±1.48 mm, 49.83±29.23%, -0.17±0.43 mm for Group A and 2.03±1.16 mm, 62.84±29.70% and -0.05±0.56 mm for Group B. Inter-group comparison of mean LBG, % BF and C-ACP revealed no statistical significant difference between the groups (p>0.05) [Table/Fig-7].
Discussion
Previous in vitro and animal studies have shown distinct effects of CsA on alveolar bone. Despite the contradicting reports, based on Four favourable studies by Fu E, Zhang DW, Horowitz MC, Ekelund AL [11,12,15,24] and a lone human trial [13], CsA was envisaged and used in the present study. In the present study, combination of CsA A with β-TCP has not shown significant CAL gain, PDR, LBG and %BF than β-TCP alone. The results of the present study are at variance with the study by Dhawan S et al., who used CsA in conjunction with DFDBM, showed a greater linear bone fill of 3.8±0.31 mm compared to DFDBM alone (3.06±0.30 mm) [13].
The lack of clinical and radiographic benefit could be attributed to no significant effect of locally administered CsA (2 mg) on periodontal regeneration. The lack of adverse reactions, such as allergies, abscesses, or rejection of the implanted materials, suggest that both the graft and the locally administered CsA were well tolerated confirming the safety of the drug.
The beneficial bone activity of CsA is reported to be dose dependent. While smaller doses enhance bone formation (0.5- 2 mg) [11–14], larger doses appear to result in bone resorption (15-30 mg). In an animal study by Movsowitz C et al., systemic administration of CsA, 7-15 mg/kg body weight resulted in improved bone formation and a 30 mg/kg body weight produced toxic skeletal demineralization [25]. In comparison, the present study used a miniscule amount of CsA (2 mg) which appears to be too low for bone enhancement and not enough to trigger bone resorption. Probably the local dosage needs to be progressively increased to a safe level and at the same time not to increase at the expense of attracting the demineralizing effect. While most of the animal studies favoured CsA action in modifying the bone metabolism, few isolated and sporadic investigations [12,13] studied its effects on combating the antigenic potential of graft materials, like tissue inflammation and foreign body reaction after DFDBM transplantation.
A Chinese study [12], stated that the methods like deep freezing, freeze drying and gamma radiation to make the allografts free from immunogenicity and disease transmission, interferes with bone healing and mechanical properties of allografts, unlike addition of CsA to allograft. Despite the limitations of the study, results showed improvement in the bone defects in both the groups, once again confirming the efficacy of carrier material used. The improvement was in accordance with the previous studies, where β-TCP treated subjects showed better results compared to open flap debridement [26].
Clinical Implications and Future Perspectives of the Study: Cyclosporine with its critical dose dependent actions and lack of any adverse effects can be taken for consideration in further preclinical research to fine tune its benefits and formulate commercially viable preparations that can be feasible in periodontal regeneration. Long-term follow-up studies with histological examination and a larger sample will be detrimental to corroborate the efficacy of cyclosporine in periodontal regeneration.
Limitation
The major limitation of the present study was lack of evidence based well documented human clinical trials supporting the beneficial effects of Cyclosporine. There was only a lone human trial [13] that could be relied on. In the attempt to evaluate the regenerative potential of cyclosporine, we had to consider previous in vitro and animal studies that have shown beneficial effects of cyclosporine on alveolar bone, considered a shortcoming. Another limitation was a six month follow up period, post the management of periodontal osseous defects treated with cyclosporine. Ideally, a follow-up period of one year is beneficial to appreciate radiographic evidence of bone fill. But considering the difficulties in handling patients in a rural background, our study was limited to a six month follow up.
Conclusion
Both the groups (β-TCP+CsA and β-TCP alone) showed statistically significant improvement in clinical and radiographic parameters, at the end of six month from the baseline. The addition of CsA to β-TCP did not provide any additional benefit for the treatment of infrabony defects. The lack of clinical benefit could be attributed to reported contradictory effects of locally administered CsA and its dose dependent action on periodontal regeneration.
S: Statistically significant (p ≤0.05)*, NS: No statistical significance (p >0.05)