Significant Haematogone Proliferation Mimicking Relapse in Acute Lymphoblastic Leukaemia on Therapy
Smeeta Gajendra1, Ruchira Misra2, Pranav Dorwal3, Rashi Sharma4, Ritesh Sachdev5
1 Associate Consultant, Department of Pathology and Laboratory Medicine, Medanta - The Medicity, Gurgaon, Haryana, India.
2 Consultant, Department of Paediatric Hemat-Oncology, Medanta - The Medicity, Gurgaon, Haryana, India.
3 Associate Consultant, Department of Pathology and Laboratory Medicine, Medanta - The Medicity, Gurgaon, Haryana, India.
4 Senior Resident, Department of Pathology and Laboratory Medicine, Medanta - The Medicity, Gurgaon, Haryana, India.
5 Senior Consultant, Department of Pathology and Laboratory Medicine, Medanta - The Medicity, Gurgaon, Haryana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Smeeta Gajendra, Department of Pathology and Laboratory Medicine, Medanta-The Medicity, Sector – 38, Gurgaon, Haryana, India.
E-mail: drsmeeta@gmail.com
Haematogones are benign B lymphoid precursors which may mimic neoplastic lymphoblasts and pose diagnostic difficulty especially when the percentage of haematogones exceeds 10% in the bone marrow. Flow cytometric analysis with combination of CD19/CD10/CD20/CD34/CD38/CD58 can be used to differentiate the two depending upon the difference in the fluorescence intensity between blasts and haematogones. We hereby present a case of Common Acute Lymphoblastic Leukaemia Associated Antigen (CALLA) positive Acute Lymphoblastic Leukaemia (ALL), in which patient presented with haematogone proliferation in bone marrow after 6 months of chemotherapy mimicking relapse. The distinction was made on flow cytometric immunophenotyping by using optimal antibody combination. Distinction of benign haematogones from neoplastic lymphoblasts is essential for disease management in cases of post chemotherapy or post marrow transplant, especially in patients of ALL. Flow cytometric immunophenotyping is reliable to distinguish haematogones from residual lymphoblasts in almost all cases when optimal antibody combinations are used.
Benign B lymphoid precursors, Flow cytometry, Minimal Residual Disease
Case Report
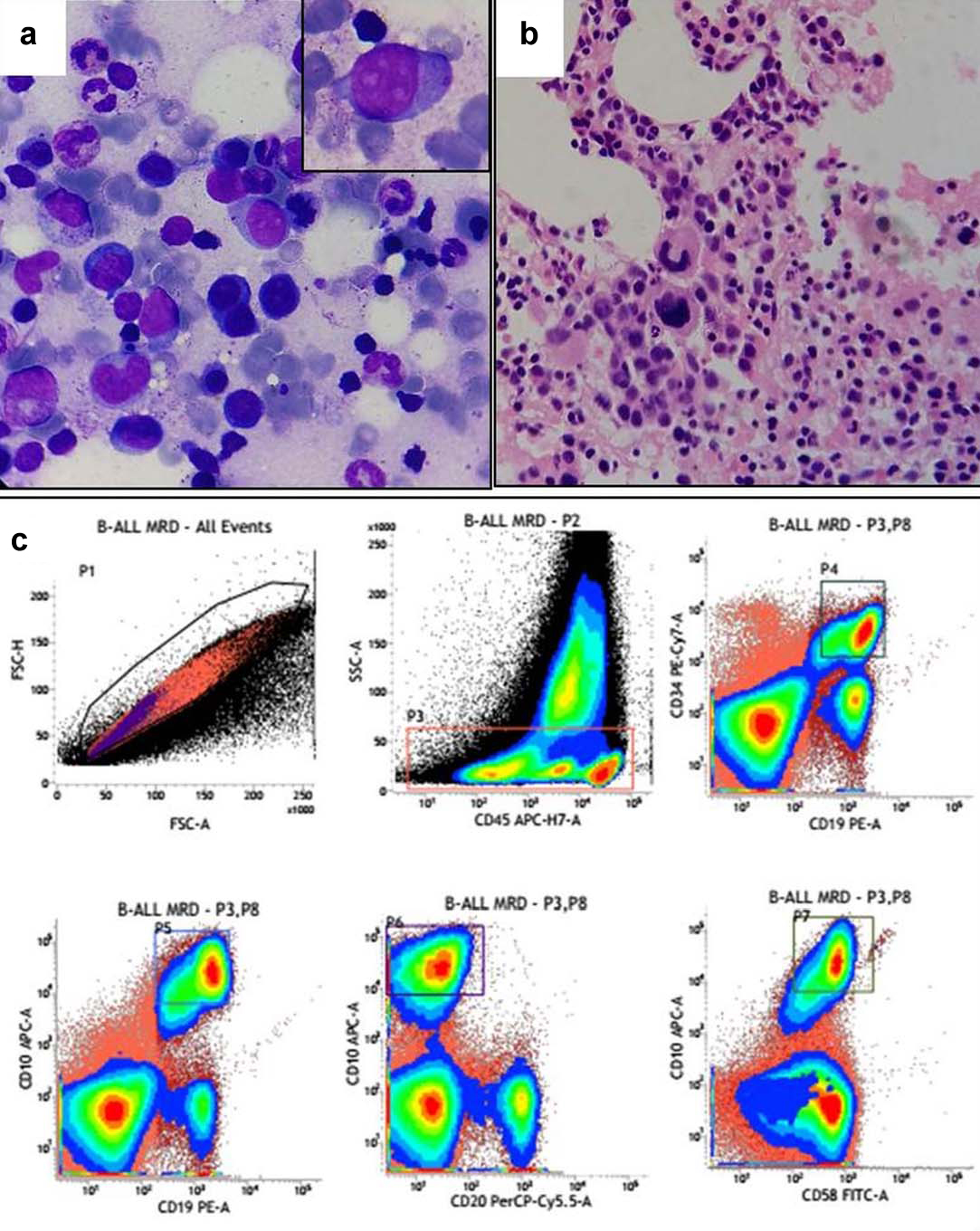
A three year-old female child presented with abdominal pain and cervical lymphadenopathy for 15 days. Peripheral blood smear showed 45% blasts which were large having high nucleo-cytoplasmic ratio, scanty basophilic cytoplasm, 1-2 inconspicuous nucleoli and few with nuclear indentation. Bone marrow aspirate revealed near total replacement by blasts (92%) [Table/Fig-1a] which were Myeloperoxidase (MPO) negative. On flow cytometric analysis [Table/Fig-2a], the blasts were positive for CD10, CD19, CD22, CD33, CD34, CD58, cCD79a, HLA-DR and negative for cCD3, CD4, CD5, CD7, CD8, CD13, CD14, CD20, CD56, CD64, CD71, CD99 and MPO. A diagnosis of Common Acute Lymphoblastic Leukaemia Associated Antigen (CALLA) positive Precursor B ALL with aberrant expression of myeloid markers (CD33) was given. Leukaemia genetic profile revealed t(12;21) (p33;q22); ETV6-RUNX1 (TEL-AML1) positivity. She was started on chemotherapy (BFM IC 2002 protocol). Bone marrow evaluation was done after one month for remission status which revealed 4% blasts and the biopsy showed normal haematopoietic elements with few small clusters of blasts. Flow cytometric evaluation for Minimal Residual Disease (MRD) was done which revealed 4% events corresponding to the blast gate defined as CD19-CD10-CD34-CD58 co-expression [Table/Fig-3]. Thus a diagnosis of B-ALL MRD positive (4%) was given.
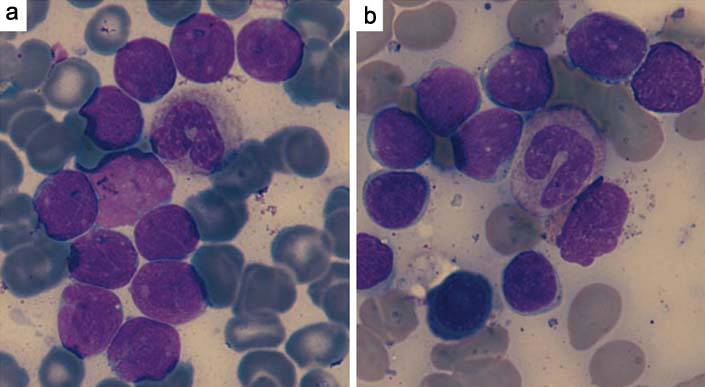
Comparison of the morphology of diagnostic lymphoblasts (a) and hematogones (b) in bone marrow smears (100X, Leishman and Giemsa stain).
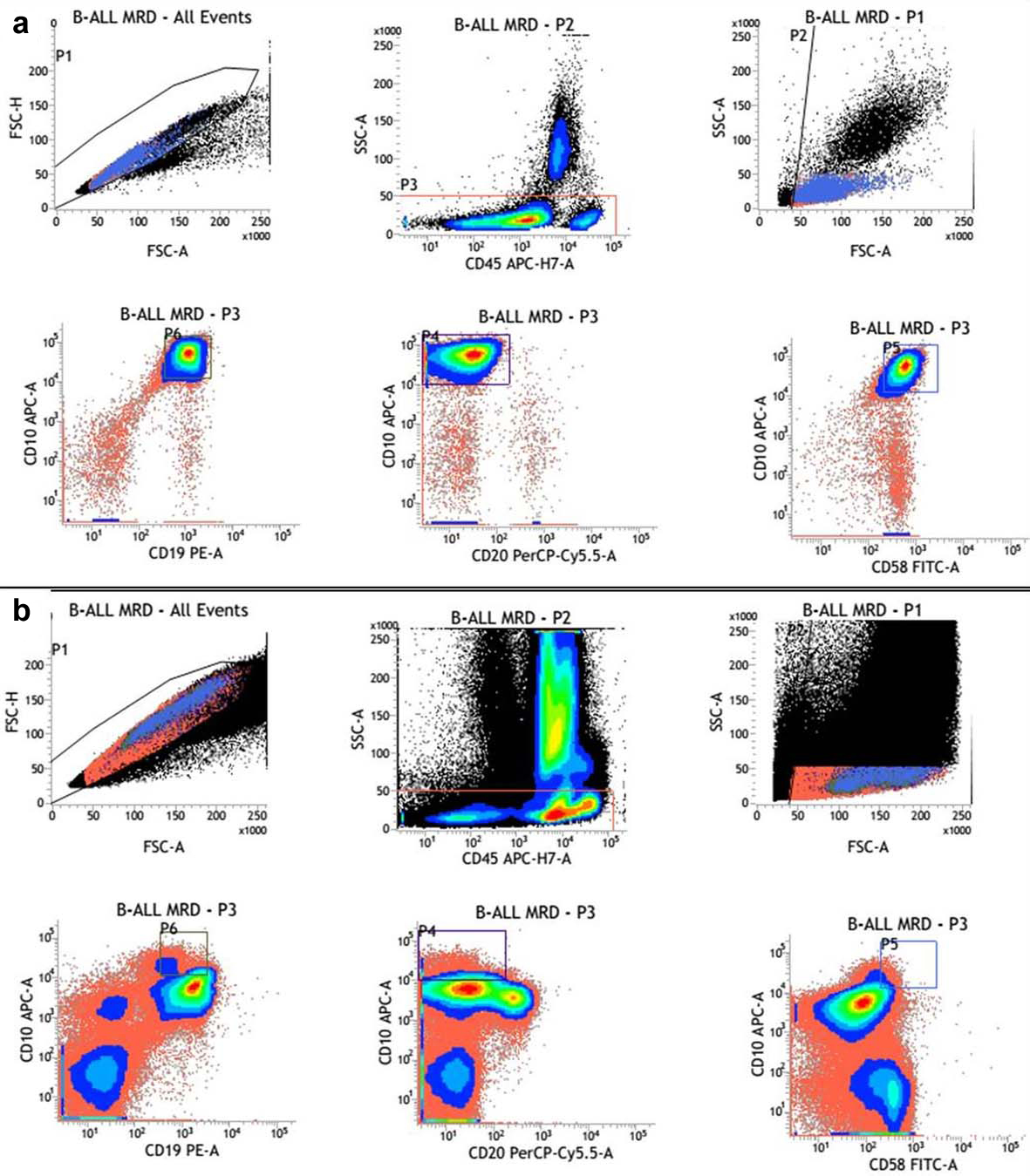
(a) Comparison of immunophenotyping of diagnostic lymphoblasts; (b) Hematogones.
(a) BM aspirate showing hematopoetic cells of all series along with few residual blast; (b) BM biopsy showing few blasts; (c) MRD analysis showing CD19-CD10-CD34-CD58 co-expressing residual blasts corresponding to diagnostic phenotype.
After 6 months bone marrow aspiration was cellular with increased lymphoid cells including mature lymphocytes to immature lymphoid cells. These immature lymphoid cells were 1-3 times the size of a small mature lymphocyte with scant agranular pale blue cytoplasm, high nucleo-cytoplasmic ratio and homogenous finely opened up chromatin [Table/Fig-1b].
A flow cytometry analysis for MRD was done which revealed 22% cells with immunophenotyping of haematogones [Table/Fig-2b]. They displayed heterogenous FSC indicating variability in size. The expression of CD10 was dimmer and heterogenous with heterogenous CD20 producing a "J- shaped trail pattern" on CD10/CD20 dot plot. They did not express CD33 which was aberrantly expressed in the blasts in the diagnostic phenotype. The diagnostic blasts showed dimmer CD45 with brighter CD10 and CD58. These cells did not express CD33 which was aberrantly expressed in the diagnostic phenotype. A MRD study using the diagnostic molecular profile of t(12:21) (p33:q22); ETV6-RUNX1 (TEL-AML1) was also negative.
After 16 months of follow up, patient is on maintenance therapy comprising of cytarabine and hydrocortisone. The patient is in complete remission.
Discussion
On morphology, it becomes difficult to differentiate haematogones, which are benign B lymphocyte precursors from the neoplastic lymphoblasts. Average number of haematogones in infants less than 2 years of age is 9%, which drops to 3.9% by 2 to 5 years and in patients more than 50 years of age, it is less than 1% [1]. The morphological distinction from neoplastic lymphoblasts is very difficult especially when the percentage of haematogones exceeds 10% in the bone marrow [2]. Flow cytometric analysis with combination of CD19/CD10/CD20/CD34/CD38/CD58 can be used to differentiate the two depending upon the difference in the fluorescence intensity between blasts and haematogones [3,4].
The increased number of haematogones have been reported in the bone marrow of children recovering from chemotherapy, regenerating marrow of treated ALL, after autologous bone marrow transplantation in acute myeloid leukaemia, aplastic conditions, other forms of bone marrow injury, infections like Cytomegalovirus, HIV and immune thrombocytopenia disorders. They pose diagnostic dilemma and potentially can be mistaken for residual disease because of their morphological similarities to neoplastic lymphoblasts. On morphology, haematogones vary in shape from 10 to 20 μ in diameter with round or oval nucleus with condensed but homogeneous chromatin. Nucleoli are absent or small and indistinct. Cytoplasm is generally scant but when present, is moderately to deeply basophilic and devoid of inclusions, granules, or vacuoles. On immunophenotyping, the leukaemic blasts are deprived of normal maturation pattern and show different pattern of antigen pattern as overexpression, underexpression and asynchronous expression [4]. The earliest B-lineage precursors express TdT, CD34, CD38, CD19, strong CD10 and dim CD22 while they lack CD20 expression (stage 1). In next stage there is loss of expression of TdT and CD34 completely and CD10 partially and up-regulation of CD20 (stage 2). Stage 3 haematogones show a progressive expression of CD20 and surface immunoglobulin light chains. Lastly CD10 is down-regulated completely, CD38 partially, and CD22 upgraded to high intensity in mature B-lymphocytes [5,6]. CD45 expression progressively increases during maturation from stage 1 to stage 3 haematogones. Neoplastic lymphoblasts may exhibit one or more aberrancies relative to normal B-lymphocyte precursors such as uniform expression of TdT and CD34; negative or under-expression of CD45, CD20, HLA-DR, and CD38; overexpression of CD10; an abnormal spectrum of CD22; and co-expression of CD34 and CD20 [6]. CD58 expression decreases as B cells mature in the bone marrow and its overexpression can be used to differentiate normal precursor B cells from precursor B-ALL blasts [7,8]. Haematogones (>5%) have now also been seen to be associated with good prognosis and increased survival rates in case of post chemotherapy or post engraftment leukaemias [9]. Most recent studies indicate a haematogones percentage of > 0.1% in bone marrow after induction phase of chemotherapy to be associated with a decreased risk of relapse and survival in cases of acute myeloid leukaemias [10].
The present case emphasizes the prognostic significance of percentage of haematogones in postchemotherapy patients of B-ALL. In our patient after the induction therapy, 22% haematogones were found and the patient is doing well after 16 months of follow up.
Conclusion
Distinction of benign haematogones from neoplastic lymphoblasts in cases of ALL is essential for disease management in cases of post chemotherapy or post marrow transplant and flow cytometry can reliably distinguish haematogones from residual lymphoblasts in almost all cases when optimal antibody combinations are used.
Conflict of interest: None declared
Funding: None.
[1]. Lucio P, Parreira A, Beemd MW Van den, Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B- ALLLeukaemia 1999 13:419-27. [Google Scholar]
[2]. Campana D, Role of Minimal Residue Disease monitoring in adult and pediatric acute lymphoblastic leukaemia Hematology/oncology clinics of North America 2009 23(5):1083-98. [Google Scholar]
[3]. Cinar SA, Kucuksezer UC, Deniz G, Implications of Minimal Residual Disease by Flow Cytometry in Pediatric Acute LeukaemiasInternational trends in immunity 2013 1(4):2326-13. [Google Scholar]
[4]. Sedak L, Busla J, Sonsala A, Twardoch M, Wieczorek M, Malinowska I, The immunophenotyping of blast cells in B-cell precursor acute lymphoblastic leukaemia: How different are they from their normal counterparts?Cytometry Part B (Clinical Cytometry) 2014 86B:329-39. [Google Scholar]
[5]. Agarwal K, Aggarwal M, Aggarwal VK, Pujani M, Nain M, Increased hematogones in an infant with bicytopenia and leucocytosis: a case reportCases J 2010 3:75 [Google Scholar]
[6]. McKenna RW, Asplund SL, Kroft SH, Immunophenotypic analysis of hematogones (B-lymphocyte precursors) and neoplastic lymphoblasts by 4-color flow cytometryLeuk Lymphoma 2004 45(2):277-85. [Google Scholar]
[7]. Lee X. RV, Braylan RC, Rimsza LM, CD58 expression decreases as nonmalignant B cells mature in bone marrow and are frequently overexpressed in adult and pediatric precursor B-cell acute lymphoblastic leukaemiaAm J Clin Pathol 2005 123(1):119-24. [Google Scholar]
[8]. Braham Jmili N, Nsaibia S, Jacob MC, Omri H, Laatiri MA, Yacoub S, Immunophenotypic analysis of bone marrow B lymphocyte precursors (hématogones) by flow cytometryClin Lab Sci 2009 22(4):208-15. [Google Scholar]
[9]. Shima T, Miyamoto T, Kikushige Y, Mori Y, Kamezaki K, Takase K, Quantitation of hematogones at the time of engraftment is a useful prognostic indicator in allogeneic hematopoietic stem cell transplantationBlood 2013 121(5):840-48. [Google Scholar]
[10]. Hassanein M, Haggag R, El Shorbagy SM, Ebian HF, Prognostic Value of Hematogones in Patients with Acute Myeloid Leukaemia in First Complete RemissionJ Blood Disord Transfus 2015 6:6 [Google Scholar]