Breast carcinoma is one of the most common malignancies affecting the female population accounting to approximately 1.67million cases diagnosed in the year 2012, coming to an estimate of 25% of all cancers [1]. It is a heterogeneous disease entity encompassing numerous distinctive histological, immunohistochemical and gene profile-based subtypes. The cell of origin of breast tumours is of utmost significance, since they hold subsequent associations with aetiology, pathogenesis and selective treatment outcomes. The most likely cell of origin for the majority of carcinomas is the Estrogen Receptor (ER) expressing luminal cell, since the majority of cancers are ER positive. ER negative carcinomas may arise from ER negative myoepithelial cells or an ER positive precursor that loses ER expression in the process of evolution of cancer [2,3].
Profiles of genetic expression have embarked on breast cancer classification surpassing traditional histological grading and subtyping. A new insight into breast cancer has become predictive of not only classification of breast tumours, but also analytical of adjuvant hormonal and chemotherapeutic treatment outcomes. Breast tumours have been thus classified into four main subtypes, such as Luminal A (Hormonal receptors (HR) +ve, HER2-ve), Luminal B (HR + ve, HER2 + ve), HER2/neu Positive (HR –ve, HER2 + ve) and Triple negative breast carcinomas (HR - ve, HER2 - ve) [2,3].
Triple Negative Breast Cancers (TNBC) are regarded as one of the malignant phenotypes, principally accounting for 12-25% of invasive breast cancers. Studies done on TNBC have demonstrated morphological features such as tumour size >20mm, increased incidence of axillary lymph node metastasis, high Nottingham Modified Bloom–Richardson (NMBR) grade, increased mitotic activity and extensive lymphovascular invasion with localized areas of necrosis [4–9].
Here, an attempt is made to study the clinicopathological features of triple negative breast carcinoma by assessing histomorphological features of triple negative breast cancer; analysing various parameters such as the age, site, tumour size, clinical features and treatment outcomes in triple negative breast cancer; and by comparing these clinicopathological features in luminal A, luminal B, Her2 positive and triple negative tumours.
Materials and Methods
We retrospectively studied 108 breast carcinoma specimens received at the Department of Pathology of a tertiary care hospital over a period of 2years i.e., between January 2013 to December 2014. The tumours, in which the immunohistochemical markers were identified, such as Estrogen Receptors (ER), Progesterone Receptors (PR) and HER2/neu markers, were included in the study. The tumours were classified as Luminal A (HR +ve, HER2/neu-ve), Luminal B (HR +ve, HER2/neu +ve), Her 2 Positive (HR -ve, HER2/neu +ve) and Triple negative breast Cancer (HR -ve, HER2/neu-ve). The clinicopathological details and histomorphological features of TNBCs were reviewed. The morphological parameters analysed were tumour size, tumour site, histological type, histological grade, lymph node status and the stage (TNM staging). The clinicopathological details, histomorphological and immunohistochemical features among the other groups were compared.
Various morphological features were analysed for their frequency, mean and median. The study was approved by Institutional Ethics Committee.
Results
In the present study, 108 cases of breast cancer were reviewed, of which 34 cases were TNBC. Out of the 34 patients, the average age at clinical presentation was 48years, with the minimum age being 25years and the maximum age being 66years. Twenty three (67.6%) tumours were stage II where the tumour was situated in the breast with axillary lymph node metastasis and 10 patients (29.5%) were Stage III, while 1 patient (2.9%) was in the early stage. Fifty percent of tumours showed lymph node metastasis. NMBR grading done on TNBC showed that most of the tumours (55.9%) were high grade followed by intermediate grade (38.2%). Only 5.9% of the tumours had a lower grade. The most common histological type was invasive ductal carcinoma NOS type (91.2%), followed by 2.9% each of Medullary and Mucinous carcinomas [Table/Fig-1].
Clinical and histopathological features of patients with Triple negative breast carcinomas.
| Parameter | | N=34 | % |
|---|
| Nuclear grade | Low | 2 | 5.9 |
| Intermediate | 13 | 38.2 |
| High | 19 | 55.9 |
| Histopathological features | Invasive ductal carcinoma | 31 | 91.2 |
| Invasive lobular carcinoma | 0 | 0 |
| Medullary carcinoma | 1 | 2.9 |
| Mucinous carcinoma | 1 | 2.9 |
| Invasive papillary carcinoma | 0 | 0 |
| Axillary Lymph node metastasis | Absent | 17 | 50 |
| Present | 17 | 50 |
| Unknown | 0 | 0 |
| Intraductal component | Absent | 2 | 5.9 |
| Present | 32 | 94.1 |
| Stage (TNM staging) | I | 1 | 2.9 |
| II | 23 | 67.6 |
| III | 10 | 29.5 |
| IV | 0 | 0 |
| DCISNuclear grade | Low | 0 | |
| Intermediate | 0 | |
| High | 1 | 2.9 |
N = total number of patients diagnosed with TNBC.
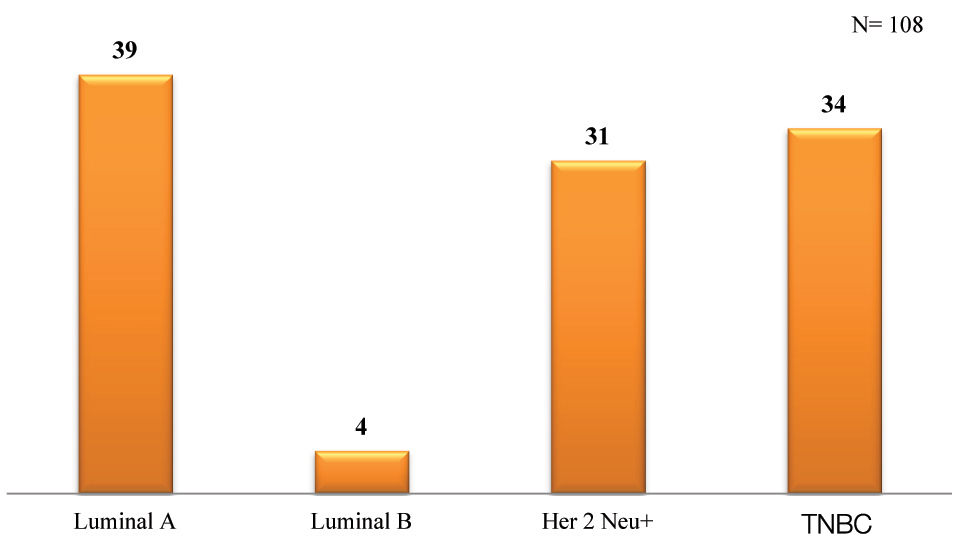
According to the study, a majority (36.1%) of the patients were diagnosed with Luminal A carcinoma, followed by TNBCs (31.5%), Her 2 Neu positive carcinomas (28.7%) and lastly Luminal B (3.7%) [Table/Fig-2].
Comparison of triple negative tumours and tumours of other subtypes in the present study.
Discussion
This is one of the studies done on the molecular subtyping of breast carcinomas in India. A quintessential highlight is made on the clinicopathological characteristics of Triple Negative Breast Carcinomas (TNBC). Thike et al., studied 61 invasive breast cancers which were profiled into molecular subtypes with expression arrays in order to illustrate specificity and sensitivity values for a constellation of immunohistochemical parameters of basal-like breast tumours [9]. Likewise, the current study revealed that immunohistochemical expression of various receptors, such as ER, PR, and Her2 Neu, which can relate to adverse pathological outcomes of the patients, which are demonstrated in the prognostic indicators, such as histological subtype, nuclear grade, NMBR Prognostic Index and lymph node status.
Of the 34 cases of TNBC, the mean age of patients was 48years. The mean age in present study was comparable to earlier similar studies [3,4,8,10]. The minimum age was 25 years in the present study, similar to the age reported by Hashmi et al., Ishikawa et al., and Qiu et al., however, genetic studies to check for familial breast carcinoma were not done [3,4,11]. Qiu et al., found a higher incidence of familial breast carcinoma in TNBC patients [11]. The maximum age in the present study was 66 years, similar to Rakha et al., in which the maximum age of TNBC patients was more than 50years [8]. In comparison with Indian studies [10–17], the present study equated to the mean age of incidence of TNBC—44-48 years of age. According to Suresh et al., with study population of 171 TNBC cases, the mean age of 49 years was similar to the current study [13]. Hence, the present study correlates to various other studies that have been conducted on the similar focus of analysis.
Of the 34 TNBC cases, it was seen that high grade (poorly differentiated) triple negative breast tumours had the highest frequency amongst all the grades according to NMBR grading scale. The present study showed 55.9% of the patients with high grade tumours, 38.2% of the patients with intermediate grade tumours, and 5.9% of the patients with low grade tumours, similar to other studies [3,4,11,13,16–20] which showed high NMBR grading [Table/Fig-3].
Comparison of nuclear grading (NMBR) of triple negative breast tumours among different studies [3,4,11,13,16–20].
| NuclearGrade | Ishikawaet al., [4]2011n=97 | Sureshet al., [13]2013n=128 | Hashmiet al., [3]2014n=205 | Fayazet al., [18]2014n=320 | Gogiaet al., [16]2014n=155 | Akhtaret al., [17]2015n=37 | Dograet al., [19] 2014n= 67 | Qiuet al., [11] 2016n= 322 | Nabiet al., [20] 2016n= 62 | Presentstudyn= 34 |
|---|
| Low(Welldifferentiated) | 2 (2.1%) | 16 (12.5%) | 10 (4.9%) | 33 (10%) | 50(56.2%) | 13(35.2%) | 1 (1.5%) | 253 (78.57%) | 3 (4.8%) | 2 (5.9%) |
| Intermediate (Moderately differentiated) | 6 (6.2%) | 34 (26.5%) | 65 (31.7%) | 105 (33%) | 19(28.4%) | 24 (38.7%) | 13 (38.2%) |
| High(Poorly differentiated) | 89 (91.8%) | 78(61%) | 130 (63.4%) | 182 (57%) | 39(43.8%) | 24 (64.8%) | 47(70.1%) | 69 (21.43%) | 35 (56.4%) | 19 (55.9%) |
Out of the 34 cases of triple negative breast tumours, 50% of the tumours showed lymph node metastasis and 50% did not show any lymph node metastasis. In this particular histopathological parameter, the present study differed from the other studies, such as Kreike et al., which showed a majority of the cases with lymph node metastasis (91.5%) [7]. The present study was somewhat similar in relation to Ishikawa et al., in which 34% of the patients had tumours with lymphovascular invasion and 62.9% of the patients had absence of lymph node metastasis [4]. This shows that typically a majority of the patients with triple negative tumours have signs of lymph node metastasis, which can lead to poor prognostic outcomes.
A plethora of histopathological parameters were analysed in the study and the predominant histologic type of TNBC was found to be infiltrating ductal carcinoma -not otherwise specified type. Similar to Hashmi et al., the study has also revealed that although ductal carcinoma was the most frequent histologic type [3], a few number of cases exhibited metaplastic and medullary like features. The current study was also similar to a study done by Thike et al., in which Infiltrating ductal carcinoma (IDC) Not otherwise specified (NOS) comprised of 92% of cases and the present study showed 91.3% of the cases [9]. Both studies showed 2-3% cases of medullary carcinomas and 1-3%, metaplastic carcinomas. All studies reported invasive ductal carcinomas (NOS) to be the predominant histologic subtype seen within all the patient populations, with invasive lobular carcinoma being the subsequent predominant histologic subtype [Table/Fig-4] [3,4,7,9,11,18–20].
Comparison of histologic subtypes of triple negative tumours among different studies [3,4,7,9,11,18–20]
| Histologic Subtype | Kreikeet al., [7](2007)n=93 | Thikeet al., [9](2010)n=653 | Ishikawaet al., [4](2011)n=97 | Hashmiet al., [3](2014)n=205 | Fayazet al., [18](2014)n=363 | Dograet al., [19](2014)n=67 | Qiu et al.,[11](2016)n=322 | Nabiet al., [20](2016)n=62 | Presentstudy(2015)n=34 |
|---|
| Invasive Ductal Carcinoma (NOS) | 81 (83%) | 606 (92%) | 92 (94.8%) | 158 (77%) | 291 (80%) | 61 (91.0%) | 204 (63.35%) | 53 (85.4%) | 31 (91.3%) |
| Apocrine carcinoma | 7 (7%) | - | 2 (2.1%) | - | - | 1 (1.5%) | - | - | - |
| Adenoid cystic carcinoma | 4 (4%) | - | - | - | - | - | - | - | - |
| Spindle cell carcinoma | - | - | 1 (1.0%) | - | - | - | - | - | - |
| Small cell carcinoma | - | - | 1 (1.0%) | - | - | - | - | - | - |
| Invasive Lobular Carcinoma | 1 (1%) | 15 (2%) | - | 5 (2.4%) | 13 (4%) | 1(1.5%) | - | 2(3.2%) | - |
| Mixed IDC and ILC | 1 (1%) | 2 (1%) | - | 1 (0.5%) | 1 (0.5%) | - | - | - | - |
| Papillary Carcinoma | - | 2 (1%) | - | 7 (3.4%) | 7 (3.4%) | - | - | - | - |
| Medullary carcinoma | - | 18 (2%) | 1 (1.0) | 12 (10.7%) | 35 (10%) | 2(3.0%) | - | - | 1 (2.9%) |
| Metaplastic Carcinoma | 3 (3%) | 9 (1%) | - | 22 (10.7%) | - | 2(3.0%) | - | - | 1 (2.9%) |
| Mucinous and tubular carcinoma | - | 1 (1%) | - | - | 9 (2%) | - | - | - | - |
| IDC (Ductal Carcinoma in situ) | - | - | - | - | - | - | - | - | 1 (2.9%) |
| Others | - | - | - | - | 12 (4%) | - | 118 (36.65%) | 7(11.2%) | - |
By comparing the tumour sizes in the current study to those of other studies [3,7–9,13,16,17], a majority of the tumours were more than 20cm in size. The present study showed that 58.8% of the patients presented with tumours more than 20cm in size and 41.2% of the tumours with less than or equal to 20cm in size, which is similar to the study done by Thike et al., which showed 70% of the tumours with more than 20cm in size [9].
In accordance to staging of triple negative tumours, a majority of the tumours were staged II and IV, however in relation to the present study; stage II was predominant amongst Indian studies. According to Suresh et al., in a study population of 128 TNBC tumours, 91 patients (71.1%) of the cases were diagnosed with stage II very similar to the present study with 23 patients (67.6%) [13]; similarly, in a study conducted by Lakshmaiah et al., 134 patients (41.7%) developed stage II TNBC, with predominantly 51.2% of stage III tumours present in cases [14].
Comparison of TNBC with Other Subtypes
Compared to triple negative tumours, luminal A tumours were typically well differentiated (low grade), with 45years being the mean age, and a majority with absent distant metastasis (61.5%). The upper outer quadrant of the breast (56.5%) and infiltrating ductal carcinoma (not otherwise specified type) was the most common histologic subtype (84.8%).
Luminal B tumours showed absence of lymphovascular invasion (75%), with stage II being the most common stage similar to TNBC and luminal A tumours, and lower NMBR grade (well-differentiation). The upper outer quadrant of the breast was involved by the tumour and infiltrating ductal carcinoma (not otherwise specified type) was the most common histologic subtype seen in all patients.
HER2/neu+ tumours have a mean age of 47years similar to TNBC tumours with the mean age being 48years. Unlike TNBC tumours, a majority of the patients with HER2+ type tumours, presented with stage III and intermediate (moderately differentiated) tumours. Like the other tumours, in the present study, they share a commonality – right upper outer quadrant is involved and IDC (NOS) is the most frequent histologic subtype involved. There is a slight difference in the ratio of lymph node metastasis (present to absent), hence lymph node metastasis is generally involved [Table/Fig-5].
Comparison of triple negative tumours and tumours of other subtypes in the present study.
| Breast CarcinomaN = 108 | Age (mean) | Tumour Size | Lymph Node Metastasis | Stage | Grade | Common Histologic Subtype | Most Common Site Involved |
|---|
| TNBCN= 34 | 48 years | >20 cm | 50% present50% absent | Stage II is the most common | High—poorly differentiated most common | IDC (NOS) type | Right upper outer quadrant |
| Luminal AN= 39 | 45 years | 2-9 cm | 38.5% present61.5% absent | Stage II is the most common | Low- (well differentiated) | IDC (NOS) | Upper outer quadrant |
| Luminal BN= 4 | 62 years | 9-12 cm | 25% present75% absent | Stage II is the most common | Low – (Well-differentiated) | IDC (NOS) | Right upper outer quadrant |
| Her 2 Neu +N= 31 | 47 years | 5-10 cm | 48.4% present51.6% absent | Stage III is the most common | Intermediate (Moderately differentiated) | IDC (NOS) | Upper outer quadrant |
All patients received similar treatment of chemotherapy i.e., 6 cycles of paclitaxel, and doxorubicin/Adriamycin, were used in combination with certain other drugs, like fluorouracil (5-FU), cyclophosphamide, and carboplatin and a majority of the patients underwent modified radical mastectomy (with axillary clearance).
The study was conclusive in analysing all the histopathological parameters. Patients, who were diagnosed with TNBC and other types of breast carcinomas, received various treatments, such as modified radical mastectomy, chemotherapeutic measures, such as 6 cycles of paclitaxel, and other medications to treat underlying infections during therapy. Most of the patients with TNBC underwent multiple treatments and cycles of chemotherapy, due to poor prognosis.
Depending on the various histopathological parameters, triple negative tumours in the current study have been compared to the clinicopathological features of tumours in other recent studies that have been conducted. It has thus proven that by comparing the age, site, histologic subtype, tumour size, lymph node status, stage, and grade, between triple negative tumours and other types have been analysed in order to determine the variations in pathological features present in patients with various types of breast carcinomas. Triple negative breast tumours have shown to have aggressive behaviour due to widespread lymphovascular invasion and distant metastasis when compared to the other subtypes.
Limitation
The limitation of the study was the absence of Fluorescent Insitu Hybridization (FISH) studies in case of 2+ Her 2 Neu positive cases. This test could not be done due to financial issues.
Conclusion
Triple negative breast carcinomas encompass a small proportion of breast cancer which shows negativity to estrogen, progesterone and human epidermal growth factor receptor 2. They are high-grade carcinomas showing nuclear pleomorphism, high mitotic rate and solid sheet like arrangement of cells. They are less likely to respond to hormonal therapy and transuzumab therapy. Hence, they are known to demonstrate poor prognosis.
N = total number of patients diagnosed with TNBC.