Introduction
Cyclosporin-A (CsA), an immunosuppressant, induces renal fibrosis and Renin Angiotensin System (RAS) is known to play a major role. CsA has the potential to increase the oxidative stress; specifically through the Advanced Oxidation Protein Products (AOPP) which could possibly stimulate fibrosis. A similar type of pathology occurs even in the gingiva known as CsA Induced Gingival Overgrowth (CIGO).
Aim
This study was undertaken to estimate the AOPP generation by Human Gingival Fibroblasts (HGF) under the influence of CsA and Angiotensin II (Ang II).
Materials and Methods
Six healthy gingival tissue samples were obtained during crown lengthening procedure and primary HGF were cultured using enzymatic digestion method. The ideal non-cytotoxic concentrations of CsA and Ang II were identified using cytotoxicity assay. Later, HGF were incubated with CsA and Ang II for 12 hours and AOPP assay was performed at zero and one hour interval.
Results
There was a statistically significant increase in AOPP production in both the CsA and Ang II when compared to the control group with a p value<0.05.
Conclusion
CsA can induce oxidative stress and preventing/controlling it may be necessary to prevent untoward effect of the drug.
Oxidative stress, Reactive oxygen species, Renin angiotensin system
Introduction
Reactive oxygen species play a major role in local inflammation and systemic diseases. Oxygen in the form of ions or peroxides form chemically reactive molecules called Reactive Oxygen Species (ROS). These are produced in the course of normal metabolism which can increase in pathological conditions. These species can bind to lipids, carbohydrates and proteins, damaging them and resulting in advanced oxidative products formation. Though many forms of protein damage are evident in in vitro study, in 1996, by Witko-Sarsat V [1] isolated dityrosine containing protein cross linking products in plasma which was designated as AOPP demonstrating a direct link to renal oxidative stress. Currently, AOPP has been identified as a major uremic toxin involved in renal diseases [2], diabetes mellitus II [3], atherosclerosis [4] and other systemic diseases. Recent studies exposed the role of AOPP in Endoplasmic Reticulum (ER) stress [5], fibrosis and other pathogenesis.
CsA, an immunosuppressant, is usually administered for renal and other organ transplants and for many dermatological conditions. This life saving drug induces a major side effect, renal fibrosis [6], which could be life threatening. Also, they can induce hepatic [7] and cardiac fibrosis [8]. The mechanism behind it’s induction of fibrosis is varied, but mostly through RAS, especially Ang II and its receptor Angiotensin Type-1 (AT1) [9]. Similar to the renal system, individuals on CsA develop gingival overgrowth known as CIGO [10]. The overgrowth though fibrotic, also have vascular features unlike overgrowth due to other drugs like phenytoin and nifedipine. Gingiva is constantly exposed to external environment; hence there is a great chance of acquiring superadded inflammation. Unlike the gingiva, kidneys are not exposed to external environment and yet they still develop fibrosis owing to the abundance of ROS, especially NADPH and AOPP suggesting drug induced oxidative stress [11]. The former has been implicated in the pathogenesis of fibrosis while the latter has been linked to ER stress and fibrosis. Both CsA and Ang II have been proposed to increase ROS production as they increase the intracellular calcium [12].
Gingival inflammation (gingivitis) is predominantly induced by biofilm and if untreated leads to an irreversible situation called periodontitis [13]. Both the conditions are infested with pro inflammatory cytokines, mediators and importantly ROS. Periodontitis not only compromises the teeth, but also been proposed as risk factor for atherosclerosis, diabetes mellitus, pre-term low birth weight babies, renal diseases etc., [14]. Being a chronic condition and with the amount of surface area, the load of bacteria and inflammatory components generated by this oral tissue will be large and sustained which makes it a strong risk factor for systemic diseases.
Inspite of numerous reports on ROS in gingivitis/periodontitis, the concept of AOPP is new to periodontium. Considering the effect of CsA and Ang II on renal ROS, especially AOPP, we were keen to observe if, without inflammation, the drug and hormone have a stimulatory effect on human gingival fibroblasts AOPP production.
Materials and Methods
This ex vivo study was carried out in Department of Periodontics, Sri Ramachandra University, Chennai, Tamil Nadu, India, over a period of six months.
Ethical Approval
This study was approved by the institutional ethics committee of Sri Ramachandra University. After proper informed consent, six patients were selected from the outpatient area of the Department of Periodontology.
Culture of Human Gingival Fibroblasts
Gingival tissue biopsy samples were obtained from six healthy volunteers undergoing crown lengthening procedure. The volunteers were selected based on the following inclusion criteria: clinically healthy gingiva of patients within the age group of 18 to 35 years and exclusion criteria: clinical signs or radiographic evidence of gingival/periodontal inflammation, systemic diseases, smoker and on antibiotics or anti-inflammatory for the past six months.
Tissue sample was collected under xylocaine local anesthesia with No: 15 BP blade. The collected samples were preserved in a transport media which contained 1X Phosphate Buffered Solution (PBS) and antibiotics (penicillin-100 IU/ml, streptomycin-100 μg/ml, and amphotericin B-100 μg/ml, gentamycin-200ug/ml, ciprofloxacin-200ug/ml) until they were processed.
Human gingival fibroblast cells were cultured using enzymatic digestion method. Monolayer cultures were incubated in complete growth essential medium at 37°C in an incubator containing humidified air (5% CO2). The cultures were observed every day and medium was replenished every two days. Once the Human Gingival Fibroblast (HGF) attained 90% confluence in culture plates they were trypsinised and sub-cultured in T-25 cm2 flask.
3-(4,5-Dimethythiazol-2-Yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) Assay
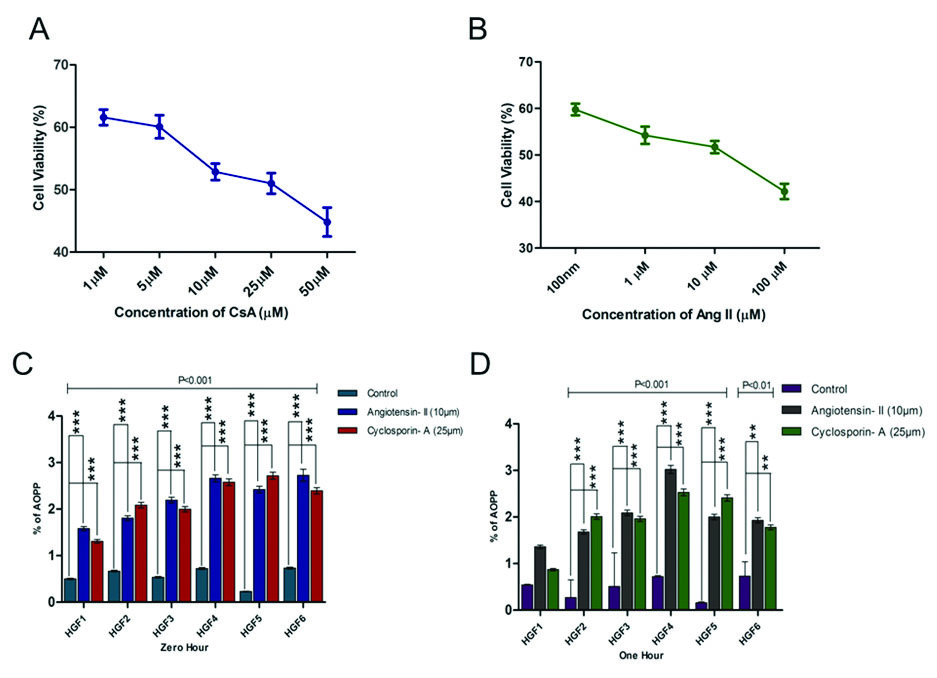
The MTT cell proliferation assay measures the cell proliferation rate and conversely, when metabolic events lead to apoptosis or necrosis, the reduction in cell viability. MTT assay was performed using the protocol given by Mossman T et al., [15]. HGF cells were seeded in 96 well plates. Then HGF cells were treated with 50 μm, 25 μm, 10 μm, 5 μm and 1 μm of CsA and 100 μm, 10 μm, 1 μm and100 nm of Ang II for 24 hours. Each of these concentrations was in triplicates. Control, diluent control and blanks wells were included. 20 μl of MTT reagent (final concentration of 0.5 mg/ml) was added to each of the wells. Then the plate was incubated at 37°C for four hours. Almost, 100 μl of Dimethyl Sulphoxide (DMSO) was added to all the wells following which the cells were re-incubated at 37°C for four hours. The plate was placed on a plate mixer for few minutes. The reading was taken at 590 nm in a Multimode plate reader (Perkin Elmer). The average values were obtained from triplicate readings. MTT readings were taken after 24 hours. Percentage of residual cell viability was determined as [1– (Optical Density of treated cells/Optical Density of control cells)] ×100. MTT assay revealed inhibitory concentration of CsA and Ang II were 25 μm and 10 μm respectively.
Sample Preparation for Advanced Oxidative Protein Products
Gingival fibroblasts were cultured in 96 well plates and cells were treated with CsA (25 μm) and Ang II (10 μm) and incubated for 12 hours following which AOPP levels were estimated. All the measurements were done in duplicates (two wells for each group).
Advanced Oxidative Protein Products Assay
Advanced Oxidative Protein Products (AOPP) was estimated using the assay put forth by Witko-Sarsat V et al., [1]. Spectrophotometry was used to estimate the production of AOPP. The assay was calibrated using chloramine-T and absorbance read at 340nm on a microplate reader at an interval of zero to one hour. AOPP concentrations were expressed as μmol/l of chloramine-T equivalents.
Statistical Analysis
The statistical significance was evaluated using Two-way ANOVA followed by Bonferroni post hoc tests. Two way ANOVA was also used to evaluate the statistical significance between zero and one hour. Software graph pad prism was used for statistical analysis. All values were expressed as mean±Standard Deviation (SD). The p value was <0.05, considered statistically significant.
Results
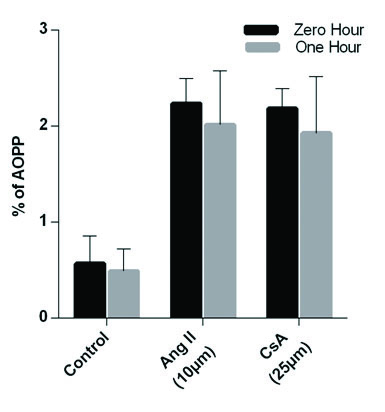
In the present study different concentration of Ang II and CsA activities against human gingival fibroblast were screened. The percentage of inhibitory properties were found to be concentration dependent, based on the Inhibitory Concentration (IC) 50 values we chose 10 μm and 25 μm concentration [Table/Fig-1a,b]. Cyclosporine and Ang II were added (zero hour and one hour incubation). One group of cells was maintained without any addition of drug or hormone which served as control group. At the end of stipulated time, culture wells were estimated for AOPP using a microtitre plate reader. There was a statistically significant increase in AOPP levels in both CsA and Ang II groups when compared to the control group (p<0.01) after zero hour and one hour [Table/Fig-1c,d]. The increase in AOPP levels was slightly higher at zero hour after the addition of CsA and Ang II when compared to one hour [Table/Fig-2]. However, the results were not statistically significant.
Effects of Ang II and CsA on human gingival fibroblast: a, b) AOPP measurements in cultured gingival fibroblast. Gingival cells at 5x103 were treated with 25 μm CsA and incubated for 12 hour at 37°C and AOPP levels were measured in both treated and control samples with indicated time levels at zero and one hour. Histogram indicated the expression level of AOPP at 10 μm of Ang II treatment at zero and one hour; c, d) Data are expressed as Mean±SEM for three independent experiments.
*p>0.05 is considered statistically significant
Graphical representation of comparison between zero and one hour.
Discussion
The present study shows significantly increased production of AOPP in human gingival fibroblasts when stimulated by CsA and Ang II. Though the production of AOPP decreased over one hour period by control cells, both cyclosporine and Ang II increased it. To our knowledge this is the first report of AOPP production by gingival cells. In recent times, AOPP has emerged as one of the most crucial reactive oxygen species. Oxidative modified proteins, AOPPs, are long-term indicators of oxidative stress, which posess pro-oxidant activity, block vasodilatation induced by Nitric Oxide (NO), induce lipid peroxidation, increase pro-inflammatory and adhesive molecules as well as cytokines [1]. Studies on the influence of cyclosporine on ROS generation are varied. CsA’s protective effect on oxidative stress could be inferred from the report of Imberti et al., [16], who observed cyclosporine preventing rat hepatocytes from lethal effect of oxidative stress. Contradicting this, Richter R et al., [17] reported CsA attenuating mitochondrial ROS preventing apoptosis /necrosis. Akool el-S et al., [18] documented that CsA induced ROS activate TGF beta gene in mesangial cells.
Animal cell and human cell culture studies have confirmed the influence, cyclosporine exhibit over Ang II generation. Navar LG et al., [19] demonstrated that there was a rise in both the circulating (plasma) and intra-renal (kidney tissue) Ang II levels in CsA-induced hypertensive rats which could be attributed to concomitant increase in renin activity. Likewise, we had demonstrated an increase in Ang II and AT1 receptors in gingiva (communicated). However, unlike Navar LG et al., we showed an increase in Angiotensin Converting Enzyme (ACE) synthesis by human gingival fibroblast. Substantiation for our findings come from a study done by Santos CF et al., [20], that detected most of the components of RAS in rat gingiva. Navar LG et al., [19] demonstrated that CsA induced hypertension is associated with elevated Ang II and ROS formation in rats and AT1 receptor blockade prevented this increase in ROS levels and development of CsA induced hypertension, suggesting that at least part of increased ROS generation is a consequence of the elevated Ang II levels and it’s not mandatory that Ang II to be involved in CsA induced ROS generation which can be substantiated in vitro by CsA stimulated increase in ROS levels in a human mesangial cells [21] and vascular endothelial cells [22]. Results of the present study show a significant increase in AOPP generation following CsA and Ang II individually, proving that both have their own capacity to generate ROS in human gingival fibroblasts. Literatures review shows that increase in intracellular calcium results in increased ROS generation [23]. In our previous study we have demonstrated increased intracellular calcium following CsA treatment in vitro [24]. The influence of CsA on calcium stores seems to vary from cell to cell.
Apart from AT1 receptor blocker, CsA-induced superoxide formation in rat aorta can be blocked by an endothelin receptor antagonist, suggesting a role of endothelin in CsA-induced ROS production [25]. It is well known that Ang II stimulates endothelin production [26]. Earlier we have demonstrated increased endothelin-1 in CsA induced human gingival overgrowth [27] suggesting more than one pathway by which cyclosporine can augment ROS generation in a tissue. Evidence of AOPPs presence in the plasma of patients with early-stage chronic kidney disease exists [28]. AOPP was found to be a more sensitive indicator of oxidative stress than ox-Low Density Lipoprotein after kidney transplantation [29]. Moreover, AOPP elevation has been reported as an independent risk factor for coronary artery disease in non-uremic patients [4]. Hence, it’s significant that the present study also demonstrates that CsA/Ang II induces AOPP production in a tissue that is highly prone for inflammation.
Limitation
The limitation of this study is that an antagonist to Ang II was not employed which would have reiterated the effect of CsA and Ang II on AOPP production. Future studies utilizing nutrients like melatonin as anti-oxidants may have important clinical implications such as restricting gingival overgrowth and controlling the risk factor for many systemic diseases by reducing the serum load of AOPP.
Conclusion
CsA and Ang II induce oxidative stress by the generation of AOPP in human gingival fibroblasts and controlling the oxidative stress may be a good option for preventing CsA induced gingival overgrowth.
[1]. Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Advanced oxidation protein products as a novel marker of oxidative stress in uremiaKidney Int 1996 49:1304-13. [Google Scholar]
[2]. Witko-Sarsat V, Gausson V, Descamps-Latscha B, Are advanced oxidation protein products potential uremic toxins?Kidney Int Suppl 2003 84:S11-14. [Google Scholar]
[3]. Taylor EL, Armstrong KR, Perrett D, Hattersley AT, Winyard PG, Optimisation of an advanced oxidation protein products assay: Its application to studies of oxidative stress in diabetes mellitusOxid Med Cell Longev 2015 2015:496271 [Google Scholar]
[4]. Kaneda H, Taguchi J, Ogasawara K, Aizawa T, Ohno M, Increased level of advanced oxidation protein products in patients with coronary artery diseaseAtherosclerosis 2002 162:221-25. [Google Scholar]
[5]. Tang X, Rong G, Bu Y, Zhang S, Zhang M, Zhang J, Advanced oxidation protein products induce hypertrophy and epithelial-to-mesenchymal transition in human proximal tubular cells through induction of endoplasmic reticulum stressCell Physiol Biochem 2015 35:816-28. [Google Scholar]
[6]. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M, Cyclosporine-associated chronic nephropathyN Engl J Med 1984 311:699-705. [Google Scholar]
[7]. Levy G, Villamil FG, Nevens F, Metselaar HJ, Clavien PA, Klintmalm Refine: A randomized trial comparing cyclosporine-A and tacrolimus on fibrosis after liver transplantation for hepatitis CAm J Transplant 2014 14:635-46. [Google Scholar]
[8]. Karch SB, Billingham ME, Cyclosporine induced myocardial fibrosis: A unique controlled case reportJ Heart Transplant 1985 4:210-12. [Google Scholar]
[9]. Naesens M, Kuypers DRJ, Sarwal M, Calcineurin inhibitor nephrotoxicityClin J Am. Soc Nephrol 2009 4:481-508. [Google Scholar]
[10]. Rateitschak-Pluss EM, Hefti A, Lortscher R, Thiel G, Initial observation that cyclosporin-A induces gingival enlargement in manJ Clin Periodontol 1983 10:237-46. [Google Scholar]
[11]. Djamali A, Reese S, Hafez O, Vidyasagar A, Jacobson L, Swain W, Nox2 is a mediator of chronic CsA nephrotoxicityAm J Transplant 2012 12:1997-2007. [Google Scholar]
[12]. Avdonin PV, Cottet-Maire F, Afanasjeva GV, Loktionova SA, Lhote P, Ruegg UT, Cyclosporine A up-regulates angiotensin II receptors and calcium responses in human vascular smooth muscle cellsKidney Int 1999 55:2407-14. [Google Scholar]
[13]. Chen C, Periodontitis as a biofilm infectionJ Calif Dent Assoc 2001 29:362-69. [Google Scholar]
[14]. Manjunath BC, Praveen K, Chandrashekar BR, Rani RMV, Bhalla A, Periodontal infections: A risk factor for various systemic diseasesNatl Med J India 2011 24:214-19. [Google Scholar]
[15]. Mosmann T, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assaysJ Immunol Methods 1983 65:55-63. [Google Scholar]
[16]. Imberti R, Nieminen AL, Herman B, Lemasters JJ, Synergism of cyclosporin A and phospholipase inhibitors in protection against lethal injury to rat hepatocytes from oxidant chemicalsRes Commun Chem Pathol Pharmacol 1992 78:27-38. [Google Scholar]
[17]. Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, Oxidants in mitochondria: From physiology to diseasesBiochim Biophys Acta 1995 1271:67-74. [Google Scholar]
[18]. Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506J Immunol 2008 181(4):2831-45. [Google Scholar]
[19]. Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A, Intrarenal angiotensin II levels in normal and hypertensive statesJ Renin Angiotensin. Aldosterone Syst 2001 2:S176-S84. [Google Scholar]
[20]. Santos C.F, Akashi A.E, Dionísio T.J, Sipert C.R, Didier D.N, Greene A.S, Characterization of a local renin-angiotensin system in rat gingival tissueJ Periodontol 2009 80(1):130-39. [Google Scholar]
[21]. Pérez de Lema G, Arribas-Gómez I, Ruiz-Ginés JA, de Arriba G, Prieto A, Rodríguez-Puyol D, Reactive oxygen species mediate the effects of cyclosporine A on human cultured mesangial cellsTransplant Proc 1997 29:1241-43. [Google Scholar]
[22]. López-Ongil S, Hernández-Perera O, Navarro-Antolín J, Pérez de Lema G, Rodríguez-Puyol M, Lamas S, Role of reactive oxygen species in the signalling cascade of cyclosporine A- Mediated up-regulation of eNOS in vascular endothelial cellsBr J Pharmacol 1998 124:447-54. [Google Scholar]
[23]. Jou MJ, Jou SB, Guo MJ, Wu HY, Peng TI, Mitochondrial reactive oxygen species generation and calcium increase induced by visible light in astrocytesAnn N Y Acad Sci 2004 1011:45-46. [Google Scholar]
[24]. Supraja A, Dinesh MG, Rajasekaran S, Balaji TM, Suresh R, Effect of cyclosporine A and angiotensin II in cytosolic calcium levels in primary human gingival fibroblastsDental Research Journal 2016 13:405-12. [Google Scholar]
[25]. Galle J, Lehmann-Bodem C, Hübner U, Heinloth A, Wanner C, CyA and OxLDL cause endothelial dysfunction in isolated arteries through endothelin-Mediated stimulation of O(2)(-) formationNephrol Dial Transplant 2000 15:339-46. [Google Scholar]
[26]. Rossi GP, Sacchetto A, Cesari M, Pessina AC, Interactions between endothelin-1 and the renin-angiotensin-aldosterone systemCardiovasc Res 1999 43:300-307. [Google Scholar]
[27]. Tamilselvan S, Raju SN, Loganathan D, Kamatchiammal S, Abraham G, Suresh R, Endothelin-1 and its receptors ET(A) and ET(B) in drug-Induced gingival overgrowthJ Periodontol 2007 78:290-95. [Google Scholar]
[28]. Liu B, Hou X, Zhou Q, Tian J, Zhu P, Xu J, Detection of advanced oxidation protein products in patients with chronic kidney disease by a novel monoclonal antibodyFree Radic Res 2011 45:662-71. [Google Scholar]
[29]. Zadrazil J, Horak P, Strebl P, Krejci K, Kajabova M, Schneiderka P, In-vivo oxidized low-density lipoprotein (ox-LDL) aopp and tas after kidney transplantation: A prospective, randomized one year study comparing cyclosporine A and tacrolimus based regimentsBiomed Pap 2012 156:14-20. [Google Scholar]