Squamous cell carcinoma is defined as “a malignant epithelial neoplasm exhibiting squamous differentiation characterized by the formation of keratin and the presence of intercellular bridges”. Squamous cell carcinoma is the most common malignant neoplasm of the oral cavity. It has been shown that up to 62% of oral cancers are preceded by oral precancerous lesion like leukoplakia characterized by epithelial dysplasia histologically [1].
The pathogenesis of oral cancer is a complex process, resulting in an overall uncoupling of growth regulation and differentiation of the affected tissue. Angiogenesis is one of the factors that play an important role in tumor growth and metastasis. Angiogenesis is the ability of pre-existing vasculature to form new microvessels [2]. Angiogenesis is a multistep process, modulated by angiogenic stimulators such as Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor (TGF), Fibroblast Growth Factor (FGF), cytokines like interleukins, Tumor Necrosis Factor (TNF) and angiogenic inhibitors such as thrombospondin-1, angiostatin and endostatin. Any shift in the net balance between angiogenic stimulators and inhibitors has a profound effect on tumor growth and metastasis [2,3]. Tumor cells produce angiogenic factors that can directly trigger the endothelial cells to develop and grow towards the budding tumor. It can be modified by various triggering factors which include, hypoxia, low pH, pressure generated by proliferating cells, tumor infiltrating inflammatory cells and genetic mutations [3].
Cytokines attract and activate macrophages, mast cells and neutrophils to the tumor site, which in turn produce angiogenic factors [2]. Angiogenesis can be used as prognostic marker, by measuring tumor microvessel density and vessel area which may predict the risk of tumor development and metastasis. It can be used as novel second target for anticancer therapy rather than direct tumor cell inhibition [3].
Mast cells are large connective tissue cells, scattered along the capillaries containing numerous basophilic granules in their cytoplasm [4]. Mast cell secretory products including histamine, heparin and tryptase are responsible for proangiogenic activity of mast cells leading to migration, proliferation and differentiation of endothelial cells [5].
Hence, the present study attempts to assess the Microvessel Density (MVD), Microvessel Area (MVA) and Mast Cell Density (MCD), in severe epithelial dysplasia and Oral Squamous Cell Carcinoma (OSCC) compared to normal mucosa and correlate the role of mast cells and angiogenesis in tumor progression.
Materials And Methods
The retrospective study was undertaken in 2015 by retrieving archived records and paraffin embedded tissue blocks of previously diagnosed age and sex matched cases of oral squamous cell carcinoma and severe epithelial dysplasia of buccal mucosa from the Department of Oral Pathology and Microbiology, College of Dental Sciences, Davangere and Government Dental College, Calicut, Kerala, India. The severe dysplasia cases were diagnosed clinically as non-homogeneous leukoplakia. The oral squamous cell carcinoma cases were treated surgically by radical neck dissection and diagnosed histologically as well differentiated. None of the patients were suffering from any other systemic diseases like cardiovascular diseases, diabetes mellitus, and anaemia. As control, normal buccal mucosa specimens were obtained during surgical procedures for other purposes like vestibuloplasty, pericornitis etc. from the Department of Oral and Maxillofacial Surgery after obtaining consent from patient with approval of the ethical committee of the institution.
A total of 21 cases were selected from initial 30 cases collected, after scrutiny by 3 observers. Only cases where there was consensus on histopathological diagnosis by the 3 observers were included in the study. Out of 21 cases, 8 cases of oral squamous cell carcinoma, 8 cases of severe epithelial dysplasia and 5 normal buccal mucosa as control were taken. The details of sample size and sampling method are given in [Table/Fig-1].
Sample size and method of sampling.
| Groups | No ofsamplesstudied | Total no of fields studied | No of fields studied in each sample for MVD, MVA | No of fields studied in each sample for MCD |
|---|
| Normal | 5 | 15 | 3 | 3 |
| Severe dysplasia | 8 | 24 | 3 | 3 |
| OSCC | 8 | 24 | 3 | 3 |
Procedure: Four to five serial sections of 5μ thickness were taken from formalin fixed paraffin embedded tissues using soft tissue microtome (Leica RM 2165, Germany). These consecutive sections of each case were stained employing Haemotoxylin and Eosin (H&E), immunostaining using CD-31 by Avidin Biotin complex method (Biogenex life sciences limited, California, USA) to demonstrate blood vessels and toluidine blue staining for the identification of mast cells [5].
The anti CD-31 antibody highlighted the microvessels by staining endothelial cell membrane. Any cluster of endothelial cells that was clearly separated from adjacent microvessel was considered a vessel. Differentiation of blood and lymphatic vessels was possible as lymphatic endothelial cells were devoid of brown staining.
Mast cells were stained purple while the nuclei were stained blue with toluidine blue stain. Both intact and degranulated mast cells were recognized by the organization of purple colored granules. The mast cells were spotted throughout the connective tissue with some near to or adhered to the blood vessels. Only mast cells found in areas of high vascularity (hot spots) were counted.
Image Analysis
The H&E, CD31 and toluidine blue stained sections were observed using trinocular research microscope (Olympus BX51, Japan). From each slide, 3 microscopic fields of high vascularity (hotspots) were selected.
All the images were captured using a 3 chip CCD camera (Proview, Media Cybernetics, USA) with a 10X apochromatic objective. The resultant image on the monitor had a 500X final magnification and represented 0.168mm2 of the tissue area.
All captured images were given numbers, stored in hard disc and subsequently subjected to morphometric measurements using the tools of Image-Pro Plus software V-4.1.0.0 (Media Cybernetics, USA). The MVD, MVA, MCD were calculated using the measurement tools of the image analysis software.
Morphometric Parameters
Microvessel density: All the stained vessels (except vessels with muscular walls) present in each selected hot spot field were counted. Incomplete outline of vessels at the margins of the field were not counted. It is calculated by counting the number of vessels which were traced for measuring MVA.
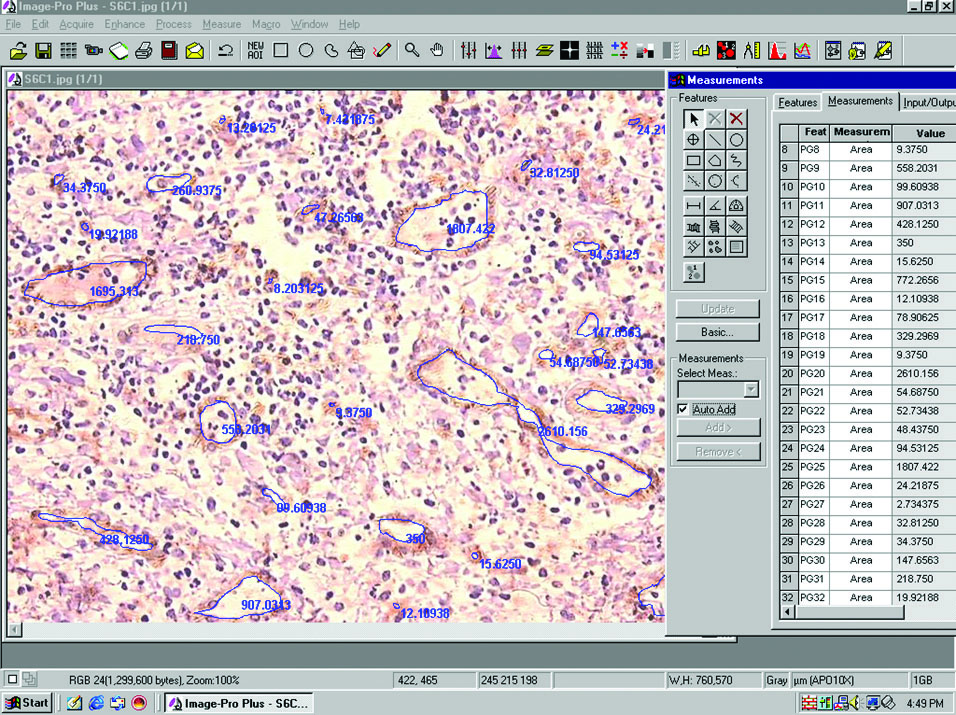
Microvessel area: It was measured in square microns. For measurements, the perimeter of the vessel lumen was traced and the software automatically calculated the area of each vessel [Table/Fig-2].
Photomicrograph showing MVA measurement using Image Pro Plus software. [CD-31 immunostaining, 20X objective].
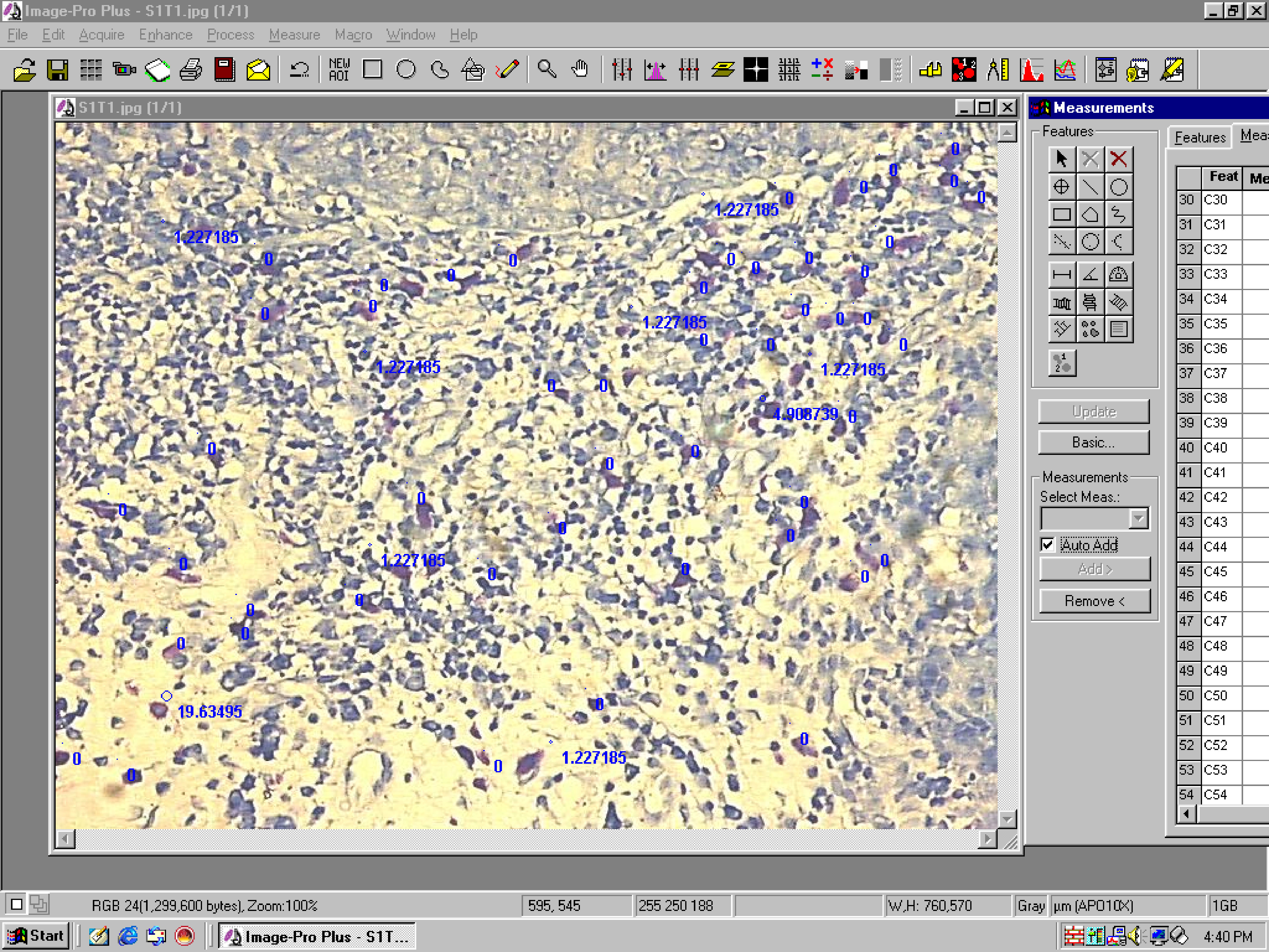
Mast cell density: All the mast cells present in the selected hot spot field were counted [Table/Fig-3]. All the data from image analysis software were exported to a master chart of the Microsoft excel. All the raw data measured per field area of 0.168mm2 was converted to square millimeter area. Further interpretations and statistical analysis was done using Microsoft excel.
Photomicrograph showing MCD counting using Image Pro Plus software. [Toluidine blue staining, 20X objective].
Statistical Analysis
The mean, standard deviation, median and range values were calculated for microvessel density, microvessel area and mast cell density. One way ANOVA (Analysis of Variance) was used for comparing the parameter for multiple groups followed by Games Howell test for pair wise comparison. A p-value of 0.05 or less was considered for statistical significance. To assess the relationship between microvessel density and mast cell density, Karl Pearson’s correlation was used. The resulting data were analyzed using software SPSS 20, IBM, Armonk, NY, United States of America.
Results
The mean with standard deviation, median, range of MVD, MVA, and MCD were calculated for normal buccal mucosa (control), severe dysplasia and OSCC.
Microvessel density (microvessels/mm2): There was increase in mean MVD from normal mucosa to severe dysplasia and OSCC. When one way ANOVA was applied for MVD, all the groups showed highly statistically significant differences (p<0.001). On application of Games-Howell test between the groups for pair wise comparison, statistically significant differences were found: Between normal mucosa and severe dysplasia, normal mucosa and OSCC, dysplasia and OSCC with p value <0.05 [Table/Fig-4].
Comparision of MVD between the groups.
| Groups | Mean MVD±SD (microvessels/mm2) | Difference between Groups |
|---|
| Groups Compared | p-value* |
|---|
| Control | 80.2±14.4 | Control - Dysplasia | <0.05,S |
| SevereDysplasia | 98.0±20.2 | Control – OSCC | <0.05,S |
| OSCC | 131.9±40.0 | Dyspalsia - OSCC | <0.01,S |
One way ANOVA, F=16.9 p<0.001, HS (Highly significant).
*Games-Howell Test, p<0.05, S (Significant)
Microvessel area (μ2/mm2): There was an increase in mean MVA from normal mucosa to severe dysplasia, but was decreased in OSCC when compared to dysplasia. When one way ANOVA was applied for MVA, all the groups showed highly statistically significant differences (p<0.001). When Games-Howell test was applied for pair wise comparison statistically significant differences were found between the groups: Between normal mucosa and dysplasia (p<0.05), between normal mucosa and OSCC (p<0.05), between dysplasia and OSCC (p<0.05) [Table/Fig-5].
Comparison of MVA between the groups.
| Groups | Mean MVA±SD (μ2/ mm2) | Difference between Groups |
|---|
| Groups Compared | p-value * |
|---|
| Control | 1696±851.9 | Control - Dysplasia | <0.05,S |
| Dysplasia | 3888.4±2323.8 | Control – OSCC | <0.05, S |
| OSCC | 2437.1±1157.4 | Dyspalsia - OSCC | <0.05, S |
One way ANOVA, F = 9.05 p<0.001, HS (Highly significant)
*Games-Howell Test, p<0.05, S (Significant)
Mast cell density (cells/mm2): There was increase in mean MCD from normal mucosa to severe dysplasia and OSCC. When one way ANOVA was applied for MCD, all the groups showed statistically significant differences (p<0.05). When Games-Howell test was applied for pair wise comparison statistically significant differences were found between the groups: Between normal mucosa and dysplasia (p<0.05), between normal mucosa and OSCC (p<0.05), between dysplasia and OSCC (p<0.05) [Table/Fig-6].
Comparison of MCD between the groups.
| Groups | Mean MCD±SD (cells2/ mm2) | Difference between Groups |
|---|
| Groups Compared | p-value * |
|---|
| Control | 39.3±19.5 | Control - Dysplasia | <0.05,S |
| Dysplasia | 103.2±74.2 | Control – OSCC | <0.05, S |
| OSCC | 143.8±75.4 | Dyspalsia - OSCC | <0.05, S |
One way ANOVA, F = 11.5 p<0.001, HS (Highly significant)
*Games-Howell test
Karl Pearson’s correlation test showed positive correlation between MVD and MCD, and MVA and MCD in severe dysplasia (r=0.61 and 0.03 respectively) and OSCC (r=0.14 and 0.09 respectively), but were not statistically significant [Table/Fig-7]. Normal tissue (control) did not show any correlation.
Karl Pearson’s correlation coefficient between MVD & MCD, MVA & MCD in the 3 groups.
| Correlation between | Control | Dysplasia | OSCC |
|---|
| R | p | r | p | r | P |
|---|
| MVD & MCD | -0.91 | 0.85 | 0.61 | 0.10 | 0.14 | 0.73 |
| MVA & MCD | -0.12 | 0.87 | 0.03 | 0.99 | 0.09 | 0.98 |
Karl Pearson’s correlation p<0.01(significant)
One way ANOVA test and Games-Howell tests were carried out by using WINPEPI software, V 11.63, Salt Lake City, Utah and Karl Pearson’s correlation test was carried out by using software SPSS 20, IBM, Armonk, NY, United States of America.
Discussion
OSCC is considered to be an aggressive epithelial neoplasm. Despite the recent advances in detection, intervention and aggressive treatment, the survival rate has improved slightly. Role of angiogenesis in neoplasms has received much attention of late and research has recommended that it can be utilized as an independent prognostic indicator for tumor development and metastasis. It has also been the target for anticancer therapy in many studies [3].
Angiogenesis means the formation of new microvessels from the pre-existing vasculature. It is the driving force for tumor growth and metastasis by providing nutrition and oxygen for metabolism. Although the tumor develops by vascularity, incorporating existing host blood vessels, but solid tumors cannot grow more than 1-2mm3 unless they develop their own network of new microvessels. Angiogenesis requires a direct or indirect role of angiogenic factors produced by tumor cells, stromal cells and inflammatory cells such as mast cells and macrophages [2].
The results in the present study suggest that MVD and MCD increase with disease progression from normal to severe dysplasia and OSCC. MVA increased from normal to dysplasia, however it decreased from dysplasia to OSCC, may be due to neovascularization of tumor tissue.
In most of the studies of angiogenesis in OSCC, MVD was the most commonly used parameter to assess vascularity [6–8]. Though vascular volume was used in few studies [6,7], none of the studies used vascular area or MVA as parameter to assess angiogenesis. In the present study, MVD and MVA were used to assess vascularity.
Preceding studies that examined the role of angiogenesis in OSCC by means of MVD reported an increase in MVD with tumor progression and lymph node metastasis, thus suggesting that MVD could be utilized as independent prognostic indicator for the same [7–11]. But some authors have detected inverse correlation between MVD and disease progression [6,12,13].
The reasons for these varying results may be due to use of different markers like CD-31, factor VIII and so on to demonstrate endothelium or due to varying methods employed in the assessment of MVD, or finally due to inter-observer variation. The varying degree of vascularization at different sites of oral mucosa and tumors arising from different sites may also lead to variations in MVD [14]. To avoid this variation, only normal buccal mucosa and severe dysplasia, OSCC of buccal mucosa were used as study groups in the present study.
The results of the present study showed increased MVD from normal to dysplasia and OSCC and are consistent with angiogenesis in tumor progression which was in accordance with the previous studies [7–11].
In the case of MVA there was an increase in values from normal to dysplasia, however from dysplasia to OSCC there was a statistically significant decrease. There were no similar studies available for direct comparison of our results on MVA. It is tempting to postulate that the reason for increased values of MVA may be because, in the early stages the increased requirement of blood supply may simply be met by an increase in vessel area. A decrease of MVA from severe dysplasia to OSCC may be due to sprouting neoangiogenesis. Fox SB et al., [15] also found a strong correlation of MVD with perimeter and a weak correlation between MVD and MVA and suggested that it could be due to shrinkage associated with fixation. Srivastava A et al., [16] found that vascular counts and percentage vascular area at tumor base in recurrence group was more than twice than in non-recurrent group. Normal keratinocytes cultured from humans secrete low levels of angiogenic stimulators and high levels of inhibitors. If keratinocytes give rise to OSCC, the tumor cells must lose their inhibitory activity or increase the production of angiogenic stimulators. Angiogenic stimulators such as VEGF, FGF, PDGF, IL-8 have been shown to be over expressed with increasing vascularity in OSCC [17]. Though strong expression of thrombospondin has been found, very little is known about angiogenic inhibitors in OSCC [18]. These angiogenic factors are not only released from tumor cells but also by host immune cells which include mast cells and macrophages [2].
Mast cells usually accumulate in surrounding newly formed capillaries in certain tumours [5]. Angiogenic factors including VEGF, bFGF produced by tumor cells stimulate mast cell migration to tumor site [19]. Mast cells play a significant role in promoting tumor angiogenesis, by secreting several potent angiogenic factors including several histamine, heparin, VEGF, bFGF, tryptase. Tryptase directly induce endothelial proliferation and mast cells act at the site of new vessel formation by secreting tryptase [20]. Intra-tumoral tryptase positive mast cells (MCT), may stimulate angiogenesis, peri-tumoral tryptase and chymase mast cells (MCTC) may promote extracellular matrix degradation and tumor progression at the invasion front [21].
In the present study, positive correlation was found between MCD and MVD and between MCD and MVA in dysplasia and OSCC. These results suggest that mast cells play a significant role in angiogenesis.
The results of present study were similar to previous studies by Macluskey M et al., on OSCC [22], Tomita M et al., on lung cancer [23], Elpek GO et al., on SCC of oesophagus [24] and Ranierri et al., study on OSCC [25] where MVD and MCD were not statistically significant in OSCC and nondysplastic leukoplakia, but a positive correlation was found between MVD and MCD within the groups. A direct correlation between mast cell counts and prognosis in pulmonary adenocarcinoma was found indicating that mast cells have a cytotoxic rather than angiogenic effect in tumors [23]. The reasons for these conflicting results could be, initially mast cells infiltrate tumor tissue to suppress neoplastic activity, once tumor infiltration occur infiltrating tumor cells might promote the angiogenic properties of mast cells while suppressing their cytotoxic functions, thereby leading to tumour angiogenesis [23]. Few authors have reported decrease in mast cell density with increasing tumor grade in OSCC suggesting probability of angiogenic factors other than mast cell playing role in tumor progression [26,27].
The increase of MVD suggests that angiogenesis increases with disease progression and increased MCD with MVD suggest that mast cells may up regulate angiogenesis in OSCC. Thus, MVD and MCD can be used as indicators for tumor progression.
Limitation
The present study was a pilot study with very few number of cases. Further investigations into mast cell derived angiogenic factors induced by tumor cells with large number of cases might provide a better understanding of the interaction of mast cells and tumor cells during tumorogenesis.
Conclusion
The angiogenesis increases with disease progression and mast cells may comprise an important cell population responsible for the neovascularisation of these tumors, and MVD and MCD can be used as indicators for disease progression. These findings also lead to the hypothesis that suppressing the angiogenic functions of mast cells may lead to new treatment modalities for OSCC.
One way ANOVA, F=16.9 p<0.001, HS (Highly significant).
*Games-Howell Test, p<0.05, S (Significant)
One way ANOVA, F = 9.05 p<0.001, HS (Highly significant)
*Games-Howell Test, p<0.05, S (Significant)
One way ANOVA, F = 11.5 p<0.001, HS (Highly significant)
*Games-Howell test
Karl Pearson’s correlation p<0.01(significant)