There is a strong association between STDs and HIV infection. AGW is a STD caused by HPV.
Though more than 200 subtypes of HPV are known, genomes of 100 types are sequenced [1]. HPV are 50-55 nm sized viruses that infect the squamous epithelium causing focal proliferation of epithelial cells [1]. Clinically cutaneous and non-cutaneous (mucosal) are the broad types of warts [1].
AGW present with a warty growth in peri-anal region and genital mucosa. AGW transmitted sexually affects peno-scrotal skin in male, vulvo-vaginal area, cervix in female and the pubic skin plus anal mucosa in both sexes [1]. Peri-anal warts may accompany genital warts either due to local spread of infection or due to direct contact during anal coitus. Most common subtypes of AGW are 6, 11 and18 [1]. Diagnosis of warts can be done clinically or detection of HPV antigen is done by Polymerase Chain Reaction (PCR) or by DNA hybridization [2]. AGW shows higher prevalence in patients engaging in anal coitus or Male Sex with Male (MSM) [3]. Mucosal disruption in AGW can facilitate HIV. Like other STDs, AGW is associated with immune suppression caused by HIV [4]. Amongst 630 million new HPV positive cases each year, 30 million have AGW [5]. Adolescent females are also at the risk of STDs and HIV [6]. Risks of malignant dysplasia increases if HIV and HPV infections are concurrently present [7]. One of the atypical and rare presentation of AGW is BLT which was first described by Buschke and Lowenstein in1925, is a sexually transmitted growth characterized by giant slow growing condyloma acuminatum that is locally aggressive and destructive [8]. Immuno-suppressed individuals may develop Epidermo-dysplasia Verruciformis (EV) like lesions especially on sun exposed skin [9]. Here clinical presentations and risk factors of AGW are compared in HIV positive and negative groups.
Materials and Methods
This observational, cross-sectional and comparative study was conducted over two year period from July 2014 to July 2016. Patients attended the dermatology outpatient department at the Dr. D. Y. Patil Hospital and Research Centre, Kolhapur, Maharashtra, India, with complaint or incidental finding of peri-genital swelling or mass was examined. The study was approved by the Institutional Ethics Committee and written informed consent (wherever applicable) was taken from all participants. Confidential interviews were undertaken to elicit risk factors of age, sex, type of sexuality and number of sexual partners. Based on history and complaint, patients were clinically screened for possible presence of warts and their site, size and presence of malignancy or co-STDs. All were screened for HIV by Trio-Dot testing with requisite pre- and post test counselling. Based on the HIV test result, patients (total sample 50 patients) were assigned to one of two groups of 25 patients each by simple consecutive sampling. All information was entered in the performa. Patients with ano-genital warts, falling between 15-60 years of age of either sex who gave their consent (or assent given by parents) to undergo the HIV testing and agreed to publish photographs were included. Those patients already undergoing treatment for AGW were excluded. HIV testing was done in the hospital laboratory though patients who wished to get their HIV test by “ELISA” method done from other standard laboratories were allowed to do so. Other laboratory tests like VDRL, Giemsa’s stain, Tzanck test, Urine- micro, Gram’s stain and biopsy (for histo-pathological diagnosis) were done as indicated. Results entered in ‘observational tables’. All information was transferred to the master-chart in MS-Excel-07 and analysed for statistical significance.
Statistical Analysis
Descriptive statistics was calculated by using MS-Excel-2007. All the measurable data i.e., quantitative variables were expressed in terms of their mean, Standard Deviation (SD) and category variables in terms of proportion. A p-value<0.05 was considered statistically significant. To see association of groups Chi-square test or Fisher-Exact test was used, wherever applicable.
Results
Mean age of patients in this study was (34±12.65) with males having a significantly higher age of 38±13.34 (SEM 2.618) and females having a mean age of 29.67±10.487 (SEM 2.141) (p=0.018). No significant difference was seen in HIV positive (32.84±12.34) and negative patients (35.16±13.10), (p>0.05). Four subjects were between 15-17 years of age i.e., “adolescents” (M:F ratio 1:1). Distribution of age groups in the study with HIV status was done as shown in [Table/Fig-1].
Age-wise distribution of AGW patients with HIV status.
| AgeGroups | HIV positive | Total(N=25) | HIV negative | Total(N=25) | p-value |
|---|
| Male | Female | Male | Female |
|---|
| 15-30 (n=24) | 7 | 9 | 16 | 2 | 6 | 8 | 0.02** |
| 31-45 (n=13) | 0 | 3 | 3 | 6 | 4 | 10 | 0.024** |
| 46-60 (n=13) | 6 | 0 | 6 | 5 | 2 | 7 | 0.74 |
| Total (n=50) | 13 | 12 | 25 | 13 | 12 | 25 | |
Chi-square test applied
“Chi-square” test revealed significant association between HIV status and age (χ2=6.51, p=0.03**). Significantly higher number of patients in the age groups of 15-30 years were HIV positive (adjusted residual 2.3) and between 31-45 years were HIV negative (adjusted residual 2.3).
The analysis of association between gender and HIV status was not significant. Chi-square test did not reveal significant association between gender and HIV status in the study as per [Table/Fig-2].
Gender distribution with HIV status.
| Sex | HIV Positive | HIV Negative | p-value |
|---|
| Male | 13 | 13 | p>0.05 (NS) |
| Female | 12 | 12 |
| Total | 25 | 25 | 50 |
Chi-square test applied
HIV status was studied with respect to number of sexual partners. Significant association between HIV status and number of sexual partners was found (χ2=8, p<0.01), using Chi-square test. Significantly more patients with HIV positivity admitted to multiple sexual partners (92%). Subjects with single partners were significantly more likely to be HIV negative (p<0.01) [Table/Fig-3].
Number of sexual partners.
| No. of sexual Partners | HIV positive | HIV negative | p-value |
|---|
| Single (N-10) | 2 (8%) | 8 (32%) | 0.0033** |
| Multiple (N-40) | 23 (92%) | 17 (68%) | 0.0033** |
Chi-square test applied
Gender distribution with respect to number of admitted sexual partners shown. No significant association was noted between gender and number of sexual partners admitted (p>0.05) using Chi-square test [Table/Fig-4].
Number of sexual partners with respect to gender.
| Number of sexual partners | SEX (N) | HIV +VE N (%) | HIV–VE N(%) | p-value |
|---|
| Single (N=10) | Male (5) | 1(50%) | 4(50%) | P >0.05(NS) |
| Female (5) | 1(50%) | 4(50%) |
| Multiple(N=40) | Male (21) | 12(52%) | 9(53%) |
| Female (19) | 11(48%) | 8(47%) |
Chi-square test applied
The sexuality of the patients was studied with respect to their HIV status. “Z” test for proportion revealed that significantly more heterosexual patients were HIV negative while homosexual patients were more likely to be HIV positive (both p<0.05) [Table/Fig-5].
Type of sexuality and HIV status.
| Type of Sexuality | HIV Positive | HIV Negative | Total | p-value |
|---|
| Heterosexual | 16 | 22 | 38 | 0.046** |
| Homosexual (Passive) | 6 | 1 | 7 | 0.041** |
| Bi-sexual | 3 | 2 | 5 | 0.63 |
| Total | 25 | 25 | 50 | |
Test for proportion applied
The distribution of warts was studied with respect to the patient’s HIV status. “Chi-square” testing reveals significant association between HIV status and anal warts (p<0.01). No significant difference in distribution of genital or extra-genital warts was noted. Extra-genital warts were commoner in HIV positive patients [Table/Fig-6].
Site of warts with HIV status
| Presentation of AGW | HIV Status | χ2 and p-value |
|---|
| Positive | Negative |
|---|
| Anal Warts | Present | 14* | 3 | χ2 =10.78p=0.001 |
| Absent | 11 | 22 |
| Genital Warts | Peno-scrotal warts | 13 | 13 | NS |
| Vulvo-vaginal warts | 12 | 12 |
| Extra genital | Present | 10 | 5 | NS |
| Absent | 15 | 20 |
| *Of these 14 patients, two patients had BLT |
Chi-square test applied
The presence of other STDs in AGW patients were analyzed and no significant association was noted with any other specific STD and HIV status using the Chi-square test in [Table/Fig-7].
Other STDs found in the patients of AGW.
| Other STD | HIV Positive (N=25) | HIV Negative (N=25) | p-value |
|---|
| Genital herpes | 4 | 1 | p> 0.05(not significant) |
| Genital molluscum | 3 | 1 |
| Secondary syphilis | 3 | 1 |
| Chancroid | 2 | 1 |
| Gonorrhoea | 3 | 1 |
| Vaginal candidiasis | 4 | 1 |
Chi-Square test applied
Discussion
AGWs or condylomata acuminata were studied in HIV positive and negative patients. Patel H et al., in India, in 2013 found overall incidence of AGWs ranged from 160 to 289 per 100,000, with a median of 194.5 per 100,000 [10]. Risk factors and clinical presentations as mentioned in methodology are discussed below.
Age: Mean age of patients in this study reflects the expected predilection of STDs for the youth. However, the mean age of males in this study (38 years) was less when compared to Caio C et al., who found mean age of males in their study 44.6±9.6 years [5]. A previous study has shown that adolescents are capable of sexual activity with females having higher incidence of STDs compared to general population [6]. In this study, four (8%) subjects were between ages of 15-17 years. The gender distribution however was equal unlike the above mentioned study. The ‘Giant Condyloma’ BLT was present in two patients of whom one was a male adolescent (of four adolescents in the study) who was HIV positive and showed in [Table/Fig-8a] with an anal lesion [8]. The patient was a victim of sexual abuse and later developed a predilection for anal sex. This highlights the spectre of sexual abuse in our society as already recognized in developed Western societies [11].
Images of “Buschke–Lowenstein tumour’ in male. BLT on anal area of male adolescent.
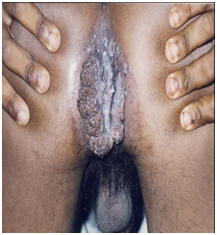
Majority of patients were in the 15-30 years age group, which is expected as this group is likely to be sexually more active and have more number of sexual partners. Maximum HIV positivity (16 out of 24 patients) is seen in this age group (p<0.05). Stevenson F et al., in a study of 847 adolescents and youths for eight years concluded that there was an acceleration of sexual risk behaviour in different races/ethnicities and genders [12].
Sex: This study did not reveal any gender predilection [Table/Fig-2] for HIV status or for number of sexual partners [Table/Fig-4] in patients with AGW. BLT was noted in one male adolescent [Table/Fig-8a] and one female [Table/Fig-8b], both testing HIV positive.
Images of “Buschke–Lowenstein tumour’ in female BLT in vulvo-vaginal area.
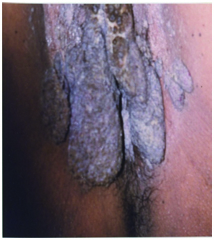
Number of sexual partners: Benard V et al., observed low income and multiple sexual relationships were the prevalent risk factors [13]. Dunne F et al., in United States found that HPV was common in females with multiple partners of the ages 20 to 24 years [14]. This study also reveals significant association of number of partners with HIV positivity [Table/Fig-3]. As high as 92% (23 out of 25), of the patients with HIV infection had multiple partners. HIV positivity in patients with single partners could reflect the sexual activities of their partner or risk factors such as blood transfusion, intravenous drug addiction or other known risk factors. Multiple partners were noted in both genders in this study [Table/Fig-4]. Majority of our patients came from the slum areas which are considered high risk areas for premarital and extramarital relations due to socioeconomic factors.
Type of sexuality: Predominant mode of transmission was heterosexual (38 out of total 50 patients) i.e., 76%. Lata E et al., found AGW is common in MSM and in heterosexual male [15]. Jiamton S et al., noted that the prevalence in MSM of AGW patients was 22.6% and 63% of them were HIV positive [16]. In this study, [Table/Fig-5] there was a significant association of HIV positivity with homosexual subjects and a negative association with heterosexual subjects.
Cutaneous (extra-genital) and anal warts: Significantly, of the 17 patients with anal warts, 14 were sero-positive (p<0.01). Conversely, of HIV positive males with anal warts, only one patient (12.5%) was heterosexual. HIV positivity in anal intercourse can be explained by the thin anal mucosa, prone for injuries facilitating the HIV transmission. Cutaneous/extra-genital warts in HIV positive may be extensive due to the defect of cell mediated immunity in patients of persistent wart infection, which turns into recalcitrant warts. One of the reason of extensive spread is “Immune Reconstitution Inflammatory Syndrome (IRIS)” in sero-positive patients [17]. The warts may be so widespread because of either autoinoculation or immunesuppression. Considering the significant association of anal warts with HIV, every patient with STD must undergo a careful anal examination to identify AGW. Though extra-genial (cutaneous) warts were more in HIV positive patients (10 Vs 5) no significant association was found.
Malignant transformation: Adler DH et al., found that in HIV-positive, concurrent infection with multiple HPV genotypes increases the risk of dysplasia [7]. These patients of AGW are prone for malignancy, as their CD4+ counts fall. Presence of RNA transcripts like E6/E7 and DNA study for expression factors of HPV sub types may be used to predict malignancy in future [18]. This study revealed no patient with malignancy, a finding that may be explained by lower mean age of the subject and the cross-sectional nature of the study. Follow up of these patients and larger sample size may provide more data. The HPV subtype prevalent is also important as some are associated with malignant transformation such as 6, 11, 16 etc., [18,19].
Association with other STDs: Since AGW is a STD, so these patients harbour other STDs too. STD patients with high risk sex behaviour can be co-infected with HIV and other STDs like syphilis, vaginitis etc., [20]. In this study too, proportionally more STDs were identified in HIV positive patients though not statistically significant.
Again STDs are [Table/Fig-7] more in HIV positive versus (v/s) negative. A study in Southern India by Kumarasamy N et al., found that Genital herpes, Syphilis, Vaginitis co-exist, out of which Genital herpes is common in HIV [21]. Our study too, reveals genital HSV as a major (5 patients) co-STD.
Ano-genital warts and HIV positivity: It is well established that the number of Langerhans cells, CD4+ T-lymphocytes, macrophages, neutrophils and natural killer cells are reduced in patients infected with HIV, a fact leading to changes in local immunity and modulating HPV infection at the tissue level [22]. This positive or supportive theory of infection is borne out by this study. Associated STDs (mainly the ulcerative) is identified as one of the factor along with other causes like multiple partners, high risk behaviour, anal sex etc., that contribute to significant HIV [23].
Atypical presentations/complications: EV is an atypical presentation, while malignancy is one of complication. In this study, we have not found any such complications. These negative findings may develop in future. Two patients (both were HIV positive) of “giant size” condyloma-acuminatum i.e., BLT found in this study [8].
Limitation
A larger study of HPV patients with HPV sub-typing is needed to detect and study malignant changes in AGW. PAP (Papaniculaou) smear which is one of the side-lab test for detection of Cervical Intraepithelial Neoplasia (CIN), was not performed in this study for the female subjects.
Conclusion
Predominant mode of transmission was heterosexual i.e., 76% in this study. AGW was significantly associated with HIV positivity in patients with sexually active age group (15-30 years), number of sexual partners, same sex partners and those indulging in anal sex HIV negativity in these patients was associated with an older age group (31-45 years), single partner subjects and extra-genital or cutaneous warts. Two BLT considered as an atypical presentation, were seen both in HIV positive patients.
These findings emphasize the need to screen all HIV positive patients for AGW and vice versa. The presence of four adolescents with AGW is noteworthy with only one patient being HIV positive. Counselling about high risk behaviour amongst the younger age group is essential.
Chi-square test applied
Chi-square test applied
Chi-square test applied
Chi-square test applied
Test for proportion applied
Chi-square test applied
Chi-Square test applied