One of the most challenging aspects in periodontal therapy is the regeneration of periodontium within the furcation defect. An enormous and rapid loss of clinical attachment is seen in teeth with involvement of furcation as compared to single rooted teeth [1]. For the re-establishment of a healthy periodontal tissue, it is important to treat subgingival plaque and calculus thoroughly by their removal. But there is diminished effectiveness of the conventional treatment in cases of furcation involvement in molars [2]. A diminished efficacy of routine periodontal treatment is seen in cases of furcation involvement in molars [3]. The lack of adequate access to instrumentation and difficulty in maintaining care due to intricate furcation anatomy leads to persistence of pathogenic bacteria and thus compromised results [2].
Grade II furcation involvement presents a distinctive clinical problem as they are difficult to be managed. Various different techniques have been employed and tested over the past three decades for management of Grade II furcations [4–8]. The closure of furcation depends upon the successful regeneration of the lost attachment apparatus [2,5–8].
A high molecular weight polysaccharide, hyaluronic acid also known as hyaluronan or hyaluronate, has been studied as a promising mediator for periodontal regeneration recently. It has a significant role in the mineralized and non-mineralized periodontal tissues for the functioning of their extracellular matrices [9]. It has a multifunctional role in periodontics including stimulation of cell migration, proliferation and differentiation and acceleration of wound healing by stimulating angiogenesis. It is used in surgical procedures due to its osteoinductive potential [10]. According to Srisuwan T et al., hyaluronic acid shows great promise in the development of engineered tissues and biomaterials for a variety of biomedical needs including orthopedic, cardiovascular, pharmacologic and oncologic applications [11]. Nadiger S and Kharidi VL observed anti-inflammatory effect of Gengigel® (0.8% hyaluronic acid) for treating gingivitis [12].
Considering its various functions and properties, an attempt has been made to evaluate the efficacy of Gengigel® in the treatment of furcation defects with coronally positioned flap. Its comparison has also been made with the surgical outcome using coronally advanced flap without Gengigel®. Surgical re-entry procedure has also been performed to assess the effect of Gengigel® on furcation defect fill.
Materials and Methods
The present study was carried out as per Helsinki’s declaration [13] and with the approval of institutional ethical committee. The subjects for this randomized controlled split mouth study were selected from the outpatient department of Department of Periodontics, MM College of Dental Sciences and Research, Mullana, Ambala, Haryana, India (sample size was determined based on the pilot study conducted on six sites which were included in the study). This clinical study was conducted over a period of 18 months.
Patients of either sex with age range of 25-60 years, who were co-operative with commitment to good oral hygiene and had no contraindication to periodontal surgery and local anaesthesia, were selected for the study [14]. Patients who were diagnosed cases of chronic periodontitis (periodontal pockets > 5 mm) with a Grade II buccal or lingual furcation defect in lower or upper molars, according to the simplified classification of Hamp et al., (1975) were included in the study [15]. Smokers, alcoholics, pregnant/nursing women and patients with potential medical complications or any disorders that could affect the treatment plan and wound healing were excluded. Teeth exhibiting Grade III mobility and any known allergy/hypersensitivity to any product used in the study were also excluded.
Study material used was Gengigel® professional syringes. It is a patented range of product, containing High Molecular Weight (HMW) hyaluronic acid (as sodium hyaluronate). It is the most important proteoglycan found naturally in mucosal extracellular matrix and plays a predominant role in tissue morphogenesis, cell migration, differentiation, and adhesion [10]. Hyaluronic acid manifests its effects on diseased periodontal tissues through its anti-inflammatory and antibacterial properties [16]. Its tissue healing effects can be harnessed to use it in collaboration with the mechanical therapy for periodontitis.
A total of 20 sites with Grade II furcation defects from 10 patients were selected using random sampling technique. Patients were divided into two groups using coin toss method as - Group A and Group B.
Group A- Ten sites with Grade II furcation defect received open flap debridement, followed by placement of 0.8% Gengigel® with coronal positioning of flap.
Group B- Ten sites with Grade II furcation defect received open flap debridement with coronal positioning of flap.
Each patient was given detailed verbal and written description of risks and benefits of the treatment along with the consent to treat agreement. All the selected patients of both the groups were subjected to presurgical protocol which included collection of pretreatment records like a detailed medical and dental history, thorough clinical examination, study casts, clinical photographs and essential laboratory investigations. The clinical parameters evaluated at baseline before the periodontal therapy and at follow up visits included Plaque index-Sillness and Loe, Gingival index-Loe and Sillness, Sulcus bleeding index-Muhlemann HR and Son, Gingival marginal position from reference points to the gingival margin using UNC-15 periodontal probe, RAL from the apical end of the occlusal stent to base of defect using UNC-15 periodontal probe, Probing Pocket Depth (PPD) from gingival margin to the base of pocket using UNC-15 probe [Table/Fig-1].
Assessment of clinical parameters; a) Probing pocket depth; b) Relative clinical attachment level; c) Position of gingival margin.
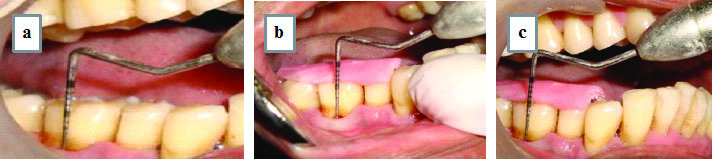
Furcation defect assessment was done in vertical and horizontal depth. Vertical Depth of Furcation Defect (VDF) was calculated by measuring the difference between the reference point (i.e., the lower border of the stent) to base of furcation and reference point to fornix of the furcation defect [2]. Horizontal Depth of Furcation Defect (HDF) from the tangent of the roots adjacent to the furcation to the horizontally deepest part of the furcation [2].
To assess the tissue changes reproducibly, measurements were taken using a UNC-15 probe to measure PPD, RAL and also to determine defect characteristics.
Surgical Protocol: After the presurgical evaluation and satisfactory response to phase I therapy, patients were subjected to surgical protocol under aseptic conditions. Each subject was comfortably seated on the dental chair before beginning the procedure. After preparing the extraoral surface with povidine iodine solution, each subject was instructed to rinse mouth using chlorhexidine digluconate solution. Xylocaine HCl was employed as anaesthetic agent for the operative region. Intracrevicular incisions using Bard Parker knife with blade no. 12 were directed to raise the flap [17]. The full thickness mucoperiosteal flap was raised 1-2 mm apical to marginal bone by using periosteal elevator. The furcation defect sites were exposed and debrided thoroughly with surgical Gracey and Universal curettes. Roots were planned and conditioned using 24% EDTA (at neutral pH) then rinsed with normal saline [Table/Fig-2].
Photograph showing surgical procedure: a) Sulcular incision; b) Defect site after debridement; c) Periosteal releasing incision; d) Root biomodification with 24% EDTA at neutral pH; e) Placement of GengigelⓇ (0.8% hyaluronic acid); f) Placement of sutures.
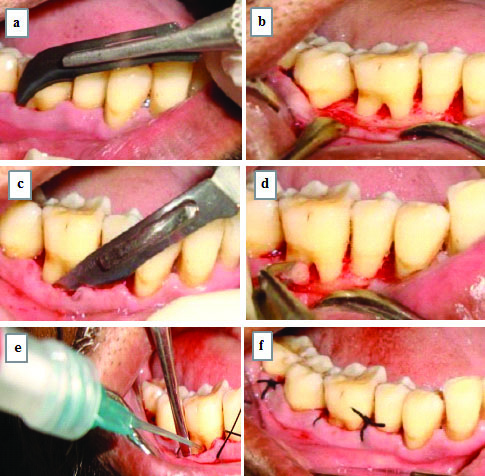
In Group A, the exposed furcation defects were thoroughly debrided and were filled by using disposable applicator with Gengigel®.
In Group B, the exposed furcation defects were mechanically debrided.
The flap was coronally positioned by periosteal releasing incision in both the groups. Sutures were given after adaptation of the flap and the surgical area was protected with non-eugenol dressing (Coe-Pack, GC America Inc, USA).
Postoperative instructions were given to the patient before leaving the dental office. All patients were prescribed systemic antibiotics, Cap Novaclox LB 500 mg three times a day for five days and a combination of Ibuprofen (400 mg) and Paracetamol (500 mg) three times a day for five days. Patients were instructed to rinse with chlorhexidine digluconate (0.2%) mouthwash twice daily for two weeks and the patients were discharged with postoperative instructions. Patients were recalled after 24 hours of surgery to evaluate the signs of postoperative complications like pain, discomfort, swelling, haematoma and haemorrhage. One week after surgery, the periodontal pack and sutures were removed and the surgical site was evaluated for the presence of local irritants. The surgical site was thoroughly irrigated with normal saline and patients were again instructed to rinse with chlorhexidine mouthwash for another eight days and were asked to gently brush the area with a soft bristle toothbrush using Charters technique. Each subject was then periodically monitored at one month, at three months and at six months postoperatively. At each of the recall visits, oral hygiene was assessed and oral hygiene instructions were reinforced.
Furcation defect fill interpretation by surgical re-entry [Table/Fig-3,4]. The following measurements were made at baseline and after six month postsurgery by surgical re-entry. The lower border of the stent was taken as Reference Point (RP).
Assessment of vertical depth as A0 - B0 and horizontal depth as H0 at baseline: a) A0=RP to BF; b) B0=RP to FF; c) H0.
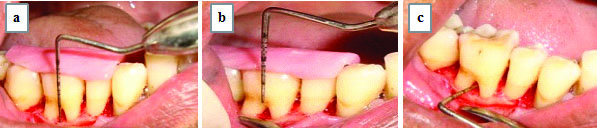
Assessment of a) Vertical depth as A6 - B6; b) Horizontal depth as H6, at six months postoperatively, through surgical re-entry.
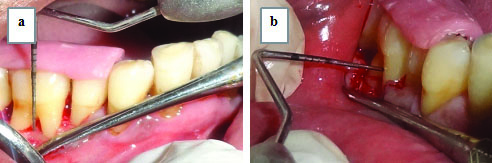
Measurements for Vertical Defect Fill of Furcation Defect:
(A0) : RP to the Base of the Furcation (BF) defect at baseline;
(B0) : RP to the Fornix of the Furcation (FF) at baseline;
(A6) : RP to the BF defect at six months postoperatively;
(B6) : RP to the FF at six months postoperatively.
Measurements for Horizontal Defect Fill of Furcation defect: From the tangent of the roots adjacent to the furcation to the horizontally deepest part of the furcation.
HD at baseline (H0);
HD at six months postoperative (H6).
Arithemetic determination for the amount of furcation defect fill: Vertical depth at baseline (A): A0 - B0;
Vertical depth at six months postoperative (B): A6 - B6;
Vertical defect fill (V): A-B;
Horizontal defect fill: H0 -H6.
All the clinical and radiological parameters recorded were subjected to the following statistical analysis:
For intragroup variations, paired t-test was performed. For comparison between the two groups/intergroup variations unpaired t-test was performed.
Results
Out of 10 patients, one patient was excluded from the study because patient did not report for follow up after the completion of therapy. Therefore, the data of eighteen sites from nine subjects were grouped and considered for statistical analysis.
clinical Parameters: The mean plaque index and gingival index scores of both the groups, at baseline and six months showed statistically highly significant and significant results, respectively. The mean gingival index score and mean bleeding index score of both groups at baseline and six months showed statistically significant results. The mean difference in probing pocket depth of both the groups, at baseline and six were statistically highly significant. The mean difference in gingival marginal position of both the groups did not show any statistically significant results whereas, the mean difference in RAL of both the groups, at baseline and six months were statistically highly significant [Table/Fig-5]. Group A and Group B did not show any significant differences for all the parameters [Table/Fig-6].
Mean changes in probing pocket depth, relative attachment level and gingival margin position in Group A and Group B.
| | PROBING POCKET DEPTH | RELATIVE ATTACHMENT LEVEL | GINGIVAL MARGINAL POSITION |
|---|
| Assessment interval | Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value | Mean±SD | Mean difference from baseline | t-value | p-value |
|---|
| GROUP A | Baseline | 3.55±0.73 | - | - | - | 10.00±1.11 | - | - | - | 6.44±.88 | - | - | - |
| Three month | 2.33±0.50 | 1.22±0.67 | 5.50 | <0.001 | 8.66±1.22 | 1.34±0.50 | 8.00 | <0.001 | 6.33±1.00 | 0.11±0.92 | 0.35 | 0.72 |
| Six month | 2.22±0.44 | 1.33±0.50 | 8.00 | <0.001 | 8.22±1.71 | 1.78±0.83 | 6.4 | <0.001 | 5.88±1.16 | 0.56±0.88 | 1.89 | 0.09 |
| GROUP B | Baseline | 3.77±0.97 | - | - | - | 10.00±1.11 | - | - | - | 6.22±0.66 | - | - | - |
| Three month | 2.11±0. 60 | 1.66±1.00 | 5.00 | <0.001 | 8.55±1.42 | 1.45±1.23 | 3.50 | <0.001 | 6.66±1.00 | 0.44±0.88 | 1.51 | 0.16 |
| Six month | 1.88±0.33 | 1.89±0.92 | 6.10 | 0.001 | 8.22±1.20 | 1.78±1.20 | 4.43 | <0.001 | 6.55±1.01 | 0.33±1.00 | 1.00 | 0.34 |
Intergroup comparisons done using unpaired ’t’ test, p-value>0.05-Non-significant, p-value<0.01-Significant, p-value<0.001-Highly significant
Comparison of mean changes in probing pocket depth, relative attachment level and gingival margin position in Group A and Group B.
| | PROBING POCKET DEPTH | RELATIVE ATTACHMENT LEVEL | GINGIVAL MARGINAL POSITION |
|---|
| Assessment interval | Groups | Mean±SD | Mean difference (A-B) | t-value | p-value | Mean±SD | Mean difference (A-B) | t-value | p-value | Mean±SD | Mean difference (A-B) | t-value | p-value |
|---|
| Baseline - Three month | Group A | 1.22±0.67 | 0.44 | 0.66 | 0.51 | 1.34±0.50 | 0.11 | 0.40 | 0.96 | 0.11±0.92 | 0.33 | 0.70 | 0.49 |
| Group B | 1.66±1.00 | 1.45±1.23 | 0.44±0.88 |
| Baseline - Six month | Group A | 1.33±0.50 | 0.55 | 1.74 | 0.10 | 1.78±0.83 | 0.00 | 0.71 | 0.94 | 0.56±0.88 | 0.23 | 1.20 | 0.21 |
| Group B | 1.88±0.92 | 1.78±1.20 | 0.33±1.00 |
Intergroup comparisons done using unpaired ‘t’ test, p-value>0.05 - Non-significant, p-value<0.01-Significant, p-value<0.001-Highly Significant
Amount of Horizontal Defect Fill: The mean difference in horizontal component of Group A at baseline and at six months, of surgical re-entry was statistically highly significant with difference in horizontal bone fill of 1.44±0.72. The mean difference in horizontal component of Group B and Group A at baseline and at six months, of surgical re-entry was statistically significant with difference in horizontal bone fill of 1.89±1.05 [Table/Fig-7].
Mean changes and comparison in amount of vertical and horizontal bone fill in Group A and Group B.
| Horizontal Component | Vertical Component |
|---|
| Assessment Time | At Baseline (Mean±SD) | At six months (Mean±SD) | Mean difference | t-value | p-value | Baseline (Mean±SD) | six months (Mean±SD) | Mean difference | t-value | p-value |
|---|
| Group A | 4.44±0.52 | 3.00±0.50 | 1.44±0.72 | 5.96 | 0.00 | 4.56±0.88 | 4.11±0.92 | 0.44±0.52 | 2.53 | 0.035 |
| Group B | 4.33±0.70 | 2.44±0.72 | 1.89±1.05 | 5.37 | 0.001 | 4.11±0.92 | 3.44±0.88 | 0.67±0.50 | 4.00 | 0.004 |
| Group A Vs Group B | - | - | 0.45 | 1.04 | 0.31 | - | - | 0.23 | 0.91 | 0.37 |
Intragroup comparisons done using paired ‘t’ test and intergroup comparisons done using unpaired ‘t’ test, p-value>0.05- Non-significant, p-value<0.01 - Significant, p-value<0.001 Highly Significant
Amount of Vertical Bone Fill: The mean difference in vertical component of Group A at baseline and six months, of surgical re-entry, was statistically significant with the mean difference in vertical bone fill of 0.44±0.52. The mean difference in vertical component of Group B at baseline and six months, of surgical re-entry was statistically significant with the mean difference in vertical bone fill of 0.67±0.50. On comparison, the mean difference in vertical and horizontal component of Group A and Group B at six months was statistically not significant [Table/Fig-7].
Discussion
Furcation areas present some of the greatest challenges to the success of periodontal therapy. The goals of therapy in furcation areas are the same as the goals in all of periodontal therapies. As the anatomy of furcation sites present with sinister situations, they are always considered as a challenge to devise appropriate treatment strategy. There comes a proposition to consider specific treatment goals for such situations. The choice of the appropriate treatment approach for a given situation depends on several factors that must be carefully evaluated prior to initiating treatment [18].
Numerous new materials have been used for the advancement of periodontal regeneration in cases of furcation involvement. The closure of the furcation is best achieved by regeneration of lost attachment apparatus which is the most desirable outcome of any furcation therapy [2]. Hyaluronic acid as a therapeutic material is used in different branches like dermatology, opthalmology and orthopaedics [19–23]. In the field of dentistry, preliminary clinical trials have been done by Vangelisti R et al., [24].
All periodontal tissues have shown the presence of hyaluron, which has been found to be particularly concentrated in the non-mineralized tissues for example gingival and periodontal ligament. It generates beneficial effects on the growth, development and repair of tissues in periodontal disease [25].
Pirnazar P et al., suggested that the clinical application of hyaluronic membrane, gels or sponges during surgical therapy reduces bacterial contamination of surgical wound site, thereby, lessening the risk of postsurgical infection and promoting more predictable regeneration [26]. Clinical studies done by Sasaki T and Kawamata-Kido H, Ballini A et al., have shown osteoinductive property of hyaluronan, as it stimulates oesteoprogenitor cells from the defect by their successive differentiation into osteoblasts and begins the formation of new bone [27,28]. Hyaloss® matrix (ester of hyaluronic acid with benzyl alcohol (HYAFF™) has been used for the correction of infrabony defects [28].
According to Hunt DR et al., hyaluronan is thought to be the best carrier for the Bone Morphogenic Proteins (BMP), the growth factors commonly documented to stimulate the formation of new bone tissue [29]. According to Koshal A et al., Gengigel® has shown significant improvements in the clinical variables of bleeding on probing and pocket depth measurements [25]. Nadiger S and Kharidi VL have observed anti-inflammatory effects of 0.2% hyaluronic acid for treating gingivitis [12]. The use of Gengigel® in addition to SRP for local subgingival treatment has been investigated clinically as well as histologically by Gontiya G et al., [30]. They observed a significant improvement in gingival parameters but periodontal factors remained unchanged. Kalra SH et al., assessed the regenerative effects of Gengigel® along with bioactive amnion membrane in a Grade II furcation area roentogenographically and found a significant improvement in defect [31]. Sandhu GK et al., used surgical re entry to assess combined approach using bioactive Gengigel® and platelet rich fibrin in Grade II furcation and found that it results in significant defect fill at six months [32].
The present study was carried out to evaluate Gengigel® in the treatment of furcation defects with coronally positioned flap.
In this study, a total of 18 sites in nine patients were selected according to the split mouth design because it excludes the influence of patient’s specific characteristics and facilitate the interpretation of trials by minimizing the effects of inter-patient variability [33].
Patients were selected on the basis of inclusion and exclusion criteria by Humagain M et al., and Taheri M et al., [2,34]. The selections of Grade II furcation were done by clinical and radiographical methods and were randomly divided into Group A and Group B. The patients selected were subjected to assessment of Plaque index (Silness J and Loe H 1964) [35], Gingival Index (Loe H and Silness J 1963) [36] and Sulcus bleeding index (Muhlemann HR and Son S 1971) [37].
The probing pocket depth, RAL and gingival margin position were recorded using UNC-15 periodontal probe with the occlusal stent [2]. These measurements were assessed at baseline, three months and six months. After the completion of phase I therapy and the attainment of surgical manageability of the tissues, the selected sites in Group A were treated surgically by open flap debridement and placement of Gengigel® with coronally positioning of flap. In Group B, the selected sites received open flap debridement with coronally positioning of flap. Systemic antibiotics and non steroidal anti-inflammatory drugs were prescribed postsurgically to control infection and patient’s discomfort [10]. Healing was uneventful without evidence of any signs and symptoms of inflammation or any postoperative complications. In all patients, oral hygiene maintenance was satisfactory at each recall visit. Hard tissue changes can be evaluated better with surgical re-entry done at six months postoperatively [18,38].
Limitation, Clinical Implications and Future Prospects
This study was carried out for a period of six months and on a small sample size. It is a short period to fully evaluate the effect of periodontal therapy particularly that utilizes the regenerative techniques. Hence, more researches with an extensive study period and greater sample size needed to be carried out to assess the long term stability of the results.
Histological evaluation was not the component of this study. This parameter should be evaluated in the future studies as it supports and confirms the findings.
Due to surgical re-entry, trauma to regenerated issue has been done. This should have been avoided using other alternative techniques like three dimensional radiography to assess the bone fill.
Conclusion
There was significant reduction in the plaque index, gingival index scores (defect site), sulcular bleeding index and probing pocket depth in both the groups, (0.8% Gengigel® with coronally positioned flap and open flap debridement with coronally positioned flap) as observed at different time intervals after the regenerative therapy. A significant gain in RAL in both the groups was observed. Gingival marginal position in Group A was shifted coronally while in Group B there was an apical shift in gingival marginal position. Surgical re-entry observations showed bone fill in horizontal and vertical component of furcation defect in both the groups but on comparison there was no statistically significant difference found. Both the groups showed improvement in clinical parameters but on comparison there was no statistically significant difference found.
From the overall observations of this study, both Gengigel® with coronally positioned flap and open flap debridement with coronally positioned flap are effective in the treatment of Grade II furcation defects. In view of the present findings, it can be concluded that the combination of Gengigel® with coronally positioned flap leads to better results in hard tissue measurement as compared to open flap debridement with coronally positioned flap.
Intergroup comparisons done using unpaired ’t’ test, p-value>0.05-Non-significant, p-value<0.01-Significant, p-value<0.001-Highly significant